A group of experts at the Dana-Farber Cancer Institute (DFCI) discovered in a new study that using two antibodies together increased their effectiveness against cancer. The duo had stronger effects than each of the two taken separately.
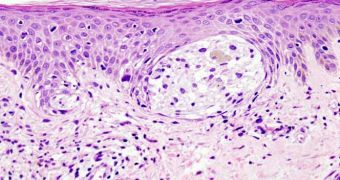
Both antibodies are targeted directly at prime survival strategies employed by cancer tumors. Phase I clinical trials indicate that administering both drugs is safe for humans, and that they are effective in addressing advanced, inoperable melanomas (skin cancers).
Ipilimumab and bevacizumab are monoclonal antibodies, which means that they are naturally-occurring, disease-fighting proteins that have had their capabilities enhanced artificially. One of the most used direction of research in cancer therapy today is augmenting the human immune system.
Our bodies have what it takes to fight cancer, at least to some extent. But tumors have the ability to suppress or elude those mechanisms. What researchers are trying to do is develop ways of getting the proteins back in the fight, but with a few aces up their sleeves.
Details of the new study will be presented today, June 4, at the annual meeting of the American Society of Clinical Oncology, which is held in Chicago. The research team carried out the clinical trials on 22 patients with metastatic melanoma, who volunteered for the task.
This is the first time investigators look at whether the two antibodies enhance or decrease each other’s effectiveness. Ipilimumab acts directly on cancer cells, by forcing the immune system to take action against them.
Its partner, bevacizumab, works by suppressing the growth of blood vessels that feed the tumors. The substance is also used to treat other types of tumors as well, such as colon, lung, and kidney cancers.
“Our findings indicate that ipilimumab and bevacizumab can be safely administered with careful management of side effects,” says the director of the DFCI Melanoma Treatment Center, F. Stephen Hodi, MD.
“The results of lab tests suggest that the two agents may work synergistically, with 14 of 21 evaluable patients experiencing a clinical benefit. This approach merits exploration in further clinical trials,” adds the expert, who was also the lead author of the new study.
Using positron emission tomography (PET) and computed tomography (CT) scans, the team determined that the duo caused a decrease in tumors, as well as a reduction in the amount of blood the cancer cells took in, Science Blog reports.
 14 DAY TRIAL //
14 DAY TRIAL //