Researchers may have just developed a complex approach for treating what is arguably the deadliest form of brain cancer. Their technique relies on a combination of radiotherapy and nanotechnology.
The main idea behind this work is to combine an extremely potent radioactive isotope with a nanoscale delivery system that would ensure extreme precision – with the goal of minimizing damage to healthy tissue surrounding the cancerous cells.
Radiotherapy has proven to be the most effective method of killing cancer cells for the past four decades. However, it has only yielded limited results against glioblastomas, a highly-aggressive form of cancer that has a near-perfect casualty rate with months of onset.
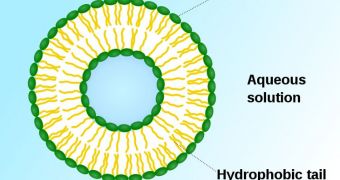
What researchers from the University of Texas Health Science Center in San Antonio (UTHSC) want to accomplish is create fat-enclosed nanoparticles capable of delivering very high doses of radiations to precisely-designated locations.
One of the main reasons why this approach is being developed is that a beam of radiation nowadays still needs to be passed through healthy brain tissue in order to reach a tumor site. Therefore, despite advancements in targeting technologies, side-effects are still a major issue.
The current situation is made even worse by the fact that exposure to even trace amounts of poorly-aimed radiation can cause severe health consequences for the patients, in addition to the cancer itself.
In the new approach, patients would receive about 20 to 30 times the currently-used dose of radiations, but the special isotope the team wants to use would only irradiate a radius of a few millimeters. The approach is detailed in the latest issue of the esteemed medical journal Neuro-Oncology, EurekAlert reports.
According to expert Andrew Brenner, MD, PhD, a CTRC neuro-oncologist and corresponding author of the study, the approach was deemed to be sufficiently effective in lab tests to merit further investigations, in human clinical trials.
“We saw that we could deliver much higher doses of radiation in animal models. We were able to give it safely and we were able to completely eradicate tumors,” Dr. Brenner explains. He will be the leader of the clinical trials as well.
The isotope rhenium-186 will be used for the trials. The substance has a very limited half-life, and it will be encapsulated in miniscule fat molecules called liposomes. These structures are about 100 nanometers across.
The delivery systems were provided by a science team led by nuclear medicine physician William T. Phillips, MD, and biochemist Beth A. Goins, PhD. Both are based at the UTHSC Department of Radiology.
“The technology is unique. Only we can load the liposomes to these very high radioactivity levels,” Dr. Brenner concludes.
 14 DAY TRIAL //
14 DAY TRIAL //