The spider silk is extremely lightweight but at the same time as strong as steel, even if its structure is based on molecular forces 100 to 1,000 times weaker than those encountered in the steel's metallic bonds, or even Kevlar's covalent bonds. A new MIT research has used theoretical modeling, laws of thermodynamics and large-scale atomistic simulation implemented on supercomputers to explain how the configuration of the silk protein creates such a strength.
"Our hope is that by understanding the mechanics of materials at the atomistic level, we will be able to one day create a guiding principle that will direct the synthesis of new materials," said lead researcher Professor Markus Buehler.
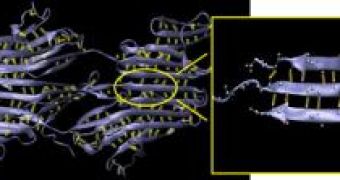
The study published in "Nano Letters" shows how the silk molecules form clusters of 3-4 hydrogen bonds that bind together stacks of short beta strands simultaneously, not sequentially, when pulled, resulting more power than if their beta strands contained just 1-2 bonds.
"Using only one or two hydrogen bonds in building a protein provides no or very little mechanical resistance, because the bonds are very weak and break almost without provocation. But using three or four bonds leads to a resistance that actually exceeds that of many metals. Using more than four bonds leads to a much-reduced resistance. The strength is maximized at three or four bonds," said Buehler, the Esther and Harold E. Edgerton Assistant Professor in the Department of Civil and Environmental Engineering.
The team determined how the external force impacted the entropic energy in the system, breaking the hydrogen bonds. Adding more hydrogen bonds in longer strands did not improve the material's strength.
"You would simply have this long chain of beta strands with lazy bonds that don't contribute to the strength of the assembly. But a material that employs many short beta strands folded and connected by three or four hydrogen bonds may exhibit strength greater than steel. In metals, the energy would be stored directly in much stronger bonds, called metallic bonds, until bonds rupture one by one. In proteins, things are more complicated due to the entropic elasticity of the noodle-like chains and the cooperative nature of the hydrogen bonds," said co-author Sinan Keten, a graduate student.
Structural proteins have mostly beta-sheets, patches folding like ribbons; short strands are placed on top of one another, at the right length to form 3-4 hydrogen bonds connecting it to the area above and beneath. Beta sheets of 3-4 hydrogen bonds make most of the molecule of beta-structured proteins, including those from muscle tissue. This means that the protein's strength has been selected during the evolution.
The other most common type is represented by alpha-helical structural proteins. Unlike proteins, steel and other synthetic materials are denser, therefore heavier.
"Metals are configured with bonds that are much stronger and require a much greater force to break. However, the crystalline lattice of a metal's structure is never perfect; it contains defects that effectively reduce the material's strength quite drastically. When you place a load on the metal, the defect can fail, possibly causing a crack to propagate. In protein's beta sheets, the confined nature of the hydrogen bond clusters helps to dissipate the energy without compromising the strength of the material. This shows the amazing ingenuity and efficiency of natural materials," said Buehler.
 14 DAY TRIAL //
14 DAY TRIAL //