One of the main things that characterize life is its predilection for improbable stuff. What living things do and what happens in them is very far from being plausible to just happen at random. The key for understanding how this improbability can come around is to understand enzymes. They are the chemicals that turn the improbable into not just probable or plausible but even reliable. Everything that happens inside us is a complex web of such highly improbable processes made reliable.
When a certain enzyme is missing, for example the enzyme known as lactase, something in the organism doesn't happen. When lactase is missing, the sugar in the milk, called lactose, cannot be broken into parts - thus, the digestion of lactose cannot take place. This is a problem encountered by certain people who are "lactose intolerant". The breaking of lactose into parts is a very improbable process by itself - but the presence of lactase makes it happen.
A bacterium like E. coli has about 1000 enzymes floating around in its cytoplasm at any moment in time. An analysis of the human genome in 2004 has found that our DNA codes for about 2700 enzymes that are used in around 900 known bioreactions. Another study this year has found that a large number of other enzymes, which are not coded by our DNA but nonetheless are needed by our metabolism, are produced by bacteria that live in our gut in a symbiosis relation with our bodies. Without them we couldn't digest carrots or apples for instance. There are around 1000 species of such symbiotic bacteria living inside us. (Read article.)
A catalyst's job
Enzymes are proteins. A protein is a long chain of amino acids (the chain is between around 100 and 1000 amino acids long). The DNA contains the information about the order in which each amino acid has to be added to the chain. As it is built, this chain of amino acids folds into a three dimensional structure which is vital for the protein's function. Not all proteins are enzymes, most aren't, but all enzymes are proteins.
What makes an enzyme special is that it is a catalyst. It isn't directly involved in the chemical reactions but it offers a space where these reactions can take place. The enzyme is a large 3D structure which has on its surface one or several places, called active sites, where other molecules that float around can bind loosely. When a molecule gets stuck to the enzyme's active site its shape is more or less deformed.
This is how the "magic" takes place. By deforming the molecule the enzyme increases the probability of certain chemicals reactions involving that molecule, reactions that otherwise would have been very unlikely (such as the decomposition of lactose). When the reaction takes place the resulting products cannot bind to the enzymes' active site and they are freed. The enzyme's active site is thus emptied and the enzyme is ready to repeat the job.
This is how highly improbable reactions are made to happen in large numbers and at great speeds over and over again. The enzymes are the pillars of complexity inside the living beings. Each cell can contain hundreds to millions of enzymes of each type, according to how important the process they catalyze is and how often the reaction is needed.
But how reliable are they?
One shouldn't think the enzymes work perfectly. In fact, sometimes, the molecule might escape from its active site before a reaction takes place, or the enzyme might not deform the molecule sufficiently. The enzyme has a certain efficiency in doing its job - it isn't an all or nothing situation.
For example, it was discovered that the most abundant enzyme in the world, the enzyme responsible for the plant's ability to have photosynthesis and to use carbon dioxide, is a highly inefficient one. This enzyme, called RuBisCO, can deal only with a few carbon dioxide molecules each second. Scientists have successfully created a mutant RuBisCO enzyme which is five times more efficient than the natural one. (In principle, such an enzyme could be used to tackle the carbon dioxide problem. Read article.)
One way nature deals with the enzymes' efficiency issue is to alter their structure in a trial and error manner gradually making the enzyme better and better. This trial and error process appears because of random mutations in the DNA, which in turn lead to small changes in the enzymes' structure. This is obviously a very slow process as most random changes don't happen to be positive. (However, it is worth noting that a random change in the DNA does not mean that the enzyme's structure is changed at random in any imaginable way. The baking analogy comes in handy here: a random change in the recipe for a cookie cannot induce any imaginable change in the cookie itself.)
Another way of increasing the enzymes' efficiency is by attaching some other molecule, called cofactor, to the enzyme. In the same way as the enzyme deforms the molecule and thus catalyzes a reaction, the cofactor deforms the enzyme and increases its ability to catalyze the reaction. But unlike the enzymes that are freed after the reaction takes place, the cofactors usually remain stuck to the enzyme. Sometimes evolution can work at a faster rate by modifying the simpler cofactors rather than the enzymes themselves.
One example of such cofactor is called NAD. This molecule binds to an enzyme called malate dehydrogenase which is also involved in photosynthesis. (Photosynthesis is a complex chemical cycle that synthesizes sugar from light, carbon dioxide and water, with oxygen as a waste product.) By binding to this enzyme some of the effects of the inefficient RuBisCO are countered making the entire cycle of photosynthesis a little less wasteful. Thus, nature instead of designing a better RuBisCO patched things up with this cofactor (as well as other like it). Moreover, NAD gets used in other processes as well - for example the metabolism of alcohol also uses it.
But not all cofactors are beneficial. Some can cause the enzyme to stop functioning properly instead of increasing its efficiency. For instance the toxic substances, known as PAHs, found in asphalts and fuels and which are the standard product of combustion from automobiles and airplanes bind to the enzymes that work on building our arteries' walls and disrupt their function. This can lead to serious illnesses. (The PAHs are also highly carcinogenic - i.e. they mess up with the DNA's reproduction by inducing mutations.)
Getting the job done: how to get from the simple to the complex
Being involved in everything that happens inside an organism, the enzymes have many functions. They make possible the cells' growth by synthesizing the needed materials; they make possible the cells' metabolism by catalyzing the decomposition of raw materials taken from the outside and producing useful components and energy; they make possible the cells' reproduction by catalyzing the DNA replication.
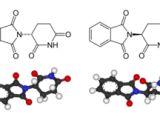
Some enzymes are highly specific - they will catalyze only one particular reaction. Some are so specific that they don't even accept both enantiomers of the same substance (enantiomers are molecules that are identical in all respects except for the fact that they are mirror images of each other). For example, most sugar molecules come in two varieties that are mirror images of each other. But all the living beings can metabolize only the right-handed molecules, leaving the others intact. Similarly, most amino acids used in living organisms (used for building their proteins) are left-handed. This bias toward only one of the two enantiomers happens because the enzymes that catalyze the reactions are so incredibly specific.
There was one highly unfortunate event that concerned this phenomenon: the story of thalidomide. One enantiomer of thalidomide is effective against morning sickness (and pregnant women have used it from 1956 to 1962), while the other enantiomer is teratogenic (it affects the development of the child in the womb and causes birth defects). About 10,000 children were born with severe malformations because their mothers had taken thalidomide during pregnancy. (The exact mechanisms in which thalidomide is involved are not yet fully understood.)
But not all enzymes are so specific. Some are specific to a certain group of molecules - they will catalyze only reactions involving molecules that contain, say, the amino or phosphate functional groups. Other enzymes are even less specific targeting only one certain type of chemical bond, regardless of the rest of the molecular structure.
Most processes in the organism involve many enzymes, each catalyzing a small part of the process. In turn, such a process involving many enzymes can be a small part in an even more complex process. Thus, there exists a highly hierarchical organization that starts with the basic reactions catalyzed by enzymes. At each level of organization even more improbable processes are turned into more than just possible but also reliable.
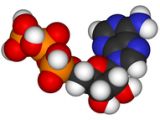
The best illustration of this idea is probably offered by ATP. Most of the energy supply in an organism comes from the reaction between a molecule called ATP and water. (In fact, after the first reaction between ATP and water there are two more reactions: the products of the first reaction, ADP and AMP, react with water producing even more energy.) This energy releasing reaction is used by virtually everything, from the synthesis of macromolecules to the transport of macromolecules across cell membranes, to the preservation of cell's structure and shape by maintaining the cytoskeleton (a structure that exists inside the cells and supports its shape), and to the muscular contractions (the muscle fibers contract when supplied with energy) needed for locomotion and respiration.
But where does the ATP come from? It doesn't come from the environment. It is synthesized by a relatively complex process involving 18 enzymes as catalysts. These enzymes are part of two cycles that work in concert to synthesize the ATP molecule. The ATP cannot be used to store the energy because it reacts with water almost immediately so it has to be produced in every place where energy is needed. Thus, the fairly complex two-cycle-18-catalysts process of synthesizing the ATP molecule is literally part of all the higher processes that need to be supplied with energy. (Energy is stored in the organism in the form of sugars - short term storage - and fats - long term storage.)
This is how the "magic" of catalysts happens. In explaining the complexity and improbability of life one can now push the problem further and wonder where do the enzymes come from? As mentioned, the information about the construction of enzymes is stored in the DNA. But the process by which that information in the DNA is transformed into proteins is also catalyzed by enzymes. Building an enzyme requires enzymes! (The details of how this happens are the subject of another article.)
The way around this paradox is to understand that a living being does not come from nothing but from other living beings. We are used at thinking about our birth in radical terms - "at time t1 I was not, at time t2 I had appeared". And people wonder where exactly is the dividing line between "I was not" and "I started being". But from the point of view of the enzymes, of the catalysts that make the whole story possible, the story line becomes blurry. The "chapters", be they individuals or species or families of species, are not so easily distinguishable. Eventually, the whole story is pushed back to the question of how life itself has appeared.
 14 DAY TRIAL //
14 DAY TRIAL //