How did inanimate chemical molecules turn into living creatures? Living beings turning into dead things is more common, but no one has ever literally witnessed the opposite process. Even when some new individual animal or plant is being born the entire process starts with a living cell. This cell has been created by another living being, and afterwards it organizes the surrounding matter into what will become the future living organism.
Thus, a new born is not really something alive that has appeared from something non-living. The only such event has been the appearance of life itself. Afterwards, there was only a continuous process of living organisms creating other living organisms. And evolution has been a process of simple organisms creating more and more complex organisms.
But, what about the origin of life as a whole? How did that happen? As I've said, no one has actually observed such a process taking place. Nonetheless, physics and chemistry offer some clues.
Empirical clues Leslie E. Orgel, a senior fellow and research professor at the Salk Institute for Biological Studies in San Diego, and whom, while at Cambridge, conducted extensive research on chemistry that may be relevant to the origin of life, explains:
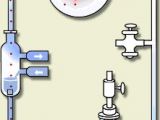
?The Miller-Urey experiment (1953) began the era of experimental prebiotic chemistry. Harold C. Urey of the University of Chicago and Stanley L. Miller, a graduate student in Urey's laboratory, wondered about the kinds of reactions that occurred when the earth was still enveloped in a reducing atmosphere. In a self-contained apparatus, Miller created such an ?atmosphere?. It consisted of methane, ammonia, water and hydrogen above an "ocean" of water. Then he subjected the gases to "lightning" in the form of a continuous electrical discharge. After a few days, he analyzed the contents of the mock ocean.
Miller found that as much as 10 percent of the carbon in the system was converted to a relatively small number of identifiable organic compounds, and up to 2 percent of the carbon went to making amino acids of the kinds that serve as constituents of proteins.
This last discovery was particularly exciting because it suggested that the amino acids needed for the construction of proteins - and for life itself - would have been abundant on the primitive planet. At the time, investigators were not yet paying much attention to the origin of nucleic acids- they were most interested in explaining how proteins appeared on the earth. [...] Similar studies provided some of the first evidence that the components of nucleic acids could have formed in the prebiotic soup as well.
In 1961, Juan Or?, then at the University of Houston, tried to determine whether amino acids could be obtained by even simpler chemistry than the Miller-Urey experiment. He mixed hydrogen cyanide and ammonia in an aqueous solution, without introducing an aldehyde. He found that amino acids could indeed be produced from these chemicals. In addition, he made an unexpected discovery: the most abundant complex molecule identified was adenine. Adenine, it will be recalled, is one of the four nitrogen-containing bases present in RNA and DNA. It is also a component of adenosine triphosphate (ATP), now the major energy-providing molecule of biochemistry. Or?'s work implied that if the atmosphere was indeed reducing, adenine - arguably one of the most essential biochemicals - would have been available to help get life started. Later studies established that the remaining nucleic acid bases could be obtained from reactions among hydrogen cyanide and two other compounds that would have formed in a reducing prebiotic atmosphere: cyanogen (C2N2) and cyanoacetylene (HC3N). Hence, early experiments seemed to indicate that under plausible prebiotic conditions, important constituents of proteins and nucleic acids could have been present on the early earth.
Strikingly, many of the same compounds generated in these various experiments have also been shown to exist in outer space. A family of amino acids that overlaps strongly with those formed in the Miller-Urey experiment has been identified in carbonaceous meteorites, along with the purine bases (adenine and guanine). Furthermore, the family of small molecules that laboratory experiments have implicated as participating in prebiotic syntheses - water, ammonia, formaldehyde, hydrogen cyanide and cyanoacetylene - is abundant in interstellar dust clouds, where new stars are born.?
Theoretical clues Besides such empirical evidence proving that complex molecules which are essential to life do appear spontaneously from simpler ones, there are two other theoretical insights regarding the mechanism behind the origin of life.
One insight comes from chemistry. A standard chemical reaction can be stated like this:
A + B -> C + D
Molecule A interacts with molecule B and gives rise to molecule C and D. A particular case of this type of reaction is when A, B, C, and D are not each a different molecule. For example one could have:
A + B -> B + D
This is called a catalytic reaction. In this reaction, B appears to have no role ? it is found both on the side of the reactants and on the side of the products. However, if one would remove B, the process A -> D will go much slower. Thus, B does play a role. For example, B may be an enzyme ? which is a very large molecule ? that slightly changes the structure of A increasing the probability it would change into D.
Finally, there may be still another particularisation: How about if the molecule D is in fact B itself?
A + B -> B + B
This is called a self-catalytic reaction. What happens here is that B increases the probability that other molecules (such as A) will change into itself. B multiplies itself.
Various such processes are well known to chemists and are one of the most exciting areas of research in chemistry. However, although molecule B multiplies itself, B is not yet a living being. B is not subjected to Darwinian evolution: although it is subjected to mutations (i.e. various factors can alter its structure), it is not subjected to natural selection. In order for natural selection to appear, B and other molecules like it must be very complex.
Why this is so: if the self-catalytic molecules are very simple, there is only a relatively small number of new molecules that can arise from them via mutations ? there is a small number of possible combinations. Thus, the available material (chemical substances like A) is sufficient for all the possible combinations to actually appear. Various self-catalytic molecules will appear in various concentrations.
However, if the self-catalytic molecules are becoming larger and larger, the number of possible combinations increases dramatically and thus the available material is no longer sufficient for all these combinations to manifest themselves. Only some of them will be lucky enough to come to existence. This is the ground on which natural selection appears. Natural selection is the expression of the fact that not all the conceivable possibilities do appear in reality ? from the set of all possibilities only a very small number of lucky ones are selected and granted the privilege of reality.
It is worth noting that molecules such as RNA and DNA as well as the simplest life forms (the viruses) are nothing else than self-catalytic large molecules.
Finally, there is an additional clue from physics. Why does complexity arise at all? Why don't things just remain simple?
Surprisingly, the process behind the origin of life and complexity in general is the same process which is also behind death and the destruction of complexity. The cause of all these things is the same: the second law of thermodynamics.
Things in general don't tend either towards complexity or simplicity; they tend towards more and more diversity and disorder. One can get disorder either by decomposing existing ordered structures, and thus obtaining a large diversity of simpler structures. Similarly, one can get disorder by combining simple structures into highly complex ones which exhibit much more chaotic and diverse behaviors than their components.
For a long time, physicists specialized in thermodynamics focused primarily on the static aspect ? on equilibrium thermodynamics ? and thus had seen only the decomposition effect. However, in the last several decades they took an increasing interest in the dynamic aspect ? the non-equilibrium thermodynamics ? and thus began observing in more and more detail the complexity-creation effect (or information growth).

 14 DAY TRIAL //
14 DAY TRIAL //