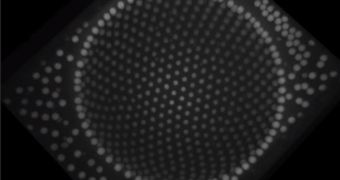
In a groundbreaking new study, researchers determined that a solution containing microscale plastic particles and oil was capable of organizing itself around the curved surface of a water droplet in a wrinkle-free manner, by forming natural pleats.
The technique has been used by seamstresses for centuries, for brining a lot of material around a narrow circumference. Pleats come in a wide variety, and they can be seen in many skirts.
Getting things to arrange themselves seamlessly around a curved surface is like trying to cover a beach ball with wrapping paper. The only way to do it is by creating laborious pleats around the ball's surface.
Apparently, the solution used in the new study is capable of creating a single-particle layer cover around a water droplet, while also producing natural pleats to smooth things out.
One of the most important applications of the new research is in understanding the physics of curved surfaces. Additionally, with the rise of nanotechnology, it is becoming increasingly important to discover ways of smoothly coating smooth surfaces.
This is required, for example, when researchers encapsulate drugs in a spherical nanoparticle, which must then be coated with tracking proteins. The molecules play an important role in detecting tumor cells, where they affix to specific biomarkers, releasing the drugs on their intended targets.
According to University of Chicago study researcher William Irvine, the conclusions of this new study may also be applied when attempting to construct mechanical devices out of nanoparticles, or when trying to determine spherical particles to self-assemble into specific structures.
In the latter case, experts need to coat the particles with something called “directional glue,” chemical markers that dictate where each of the nanoscale sphere would attach to the other. Having this ability is critical for assembling ordered structures, and not just clumps.
“Suppose you had a bunch of spheres and you wanted to glue them together. If you use something where if whenever any two surfaces touch, they glue together, you would either get a big mess or something that looks very tightly packed, like oranges in the supermarket,” the expert says.
“Taking a structure that is that small and putting directional bonds on it is very hard, unless you have a mechanism that spontaneously creates them," Irvine reveals, quoted by LiveScience.
Speaking about the new material, the expert explains: “Imagine a pleated skirt with a plaid pattern. If you looked at what happened to one of the lines from the rectangles around the pleat, you'd see that when you opened the pleat up, the lines would diverge.”
“They would no longer stay parallel to each other... [That's] exactly what we [saw in the new experiment],” he concludes.
 14 DAY TRIAL //
14 DAY TRIAL //