A team of engineers at the California Institute of Technology (Caltech) announces that it finally manged to crack the mystery of one of the most curious materials in the world. Scandium trifluoride (ScF3) is part of a class of materials that contracts when heated.
Generally, basic physics teaches us that materials tend to expand when heated up. Only a very select few materials do not display this property, and researchers have been trying to crack their mystery for quite some time now. However, most attempts in this regard have failed, until now.
The Caltech team managed to figure out how ScF3 contract when heated. That is to say, the group figured out the host of processes going on inside the material as it is subjected to high temperatures.
The researchers were led by Caltech graduate student Chen Li. Details of the work were published in the November 4 issue of the esteemed journal Physical Review Letters. The team explains that the study was informed by the need for materials that shrink under heat for a variety of applications.
For example, such materials could be used to counteract the expansion of other materials in a device, when the entire device is heated up. When the other components swell up, this one shrinks, allowing the instrument to retain its function and form at high temperatures.
“When you heat a solid, most of the heat goes into the vibrations of the atoms,” explains Brent Fultz, who holds an appointment as a professor of materials science and applied physics at Caltech. He is also one of the coauthors of the new paper.
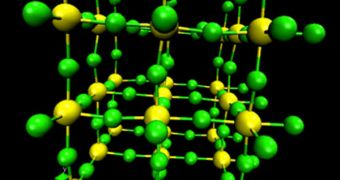
In this special class of materials, vibrations are somehow modified in such a manner that they do not lead to swelling, but rather to negative thermal expansion (shrinking). Experts decided to use ScF3 to see how this happens due to the material's simple crystal structure.
When heat is applied to the chemical, bonds between scandium and fluorine atoms tend to contract, pulling the atoms closer together. This is what led to the contraction of the material. The team also determined that a special quantum state develops in the material.
“A nearly pure quantum quartic oscillator has never been seen in atom vibrations in crystals,” Fultz explains. The studies were conducted at the Oak Ridge National Laboratory (ORNL) Spallation Neutron Source (SNS).
Using the new data, the team hopes to be able to determine how contraction occurs in other, similar materials as well. This could lead to numerous innovations in several areas of science, especially engineering and materials science.
 14 DAY TRIAL //
14 DAY TRIAL //