Understanding how basic molecules called enantiomers form in their purest state could provide us with critically important new data about the origins of life on our planet, Spanish researchers say.
A team there recently managed to conduct a new series of investigations on these molecules, which are basically one of two stereoisomers that are mirror images of each other inside amino-acids.
Stereoisomers are chains of atoms that have the exact same make-up, but which differ from each other only through their three-dimensional orientation.
The reason why studying enantiomers is so important is because they may yield new insight into the origins of chirality on our planet, which is a trait that can be summed up as handedness. Chiral molecules are non-superimposable mirror images of themselves.
The best way to understand this is by looking at a pair of hands. They are generally the same, but they cannot be neatly folded on top of one another and be identical. The orientation of the thumbs is a dead giveaway.
Similarly, molecules such as those making up amino-acids, the basic building blocks of proteins, have a certain chirality that can be either to the right or to the left. Life on Earth exhibits both left and right chirality, but in varied amounts.
Some drugs that we take are chiral, and may have an handedness that is different than that of their targets. In a bid to make more sense of how this may influence the overall effects of the therapy, the Spanish researchers conducted a complex experiment.
They were able to use complex reactions to activate a special, enatioenriched form of the basic amino-acid valine. This means that the purity of one enantiomer in the acid was artificially enhanced over the other's.
The accomplishment was made the Complutense University in Madrid, by a team of chemists led by expert Cristóbal Viedma, Chemistry World reports.
For the job, the group selected to use a non-racemic form of valine. This meant that the amino-acid did not contain an equal amounts of left- and right-handed enantiomers.
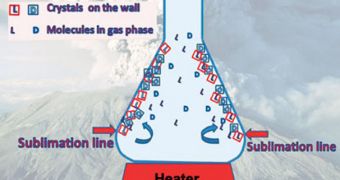
In the experiments, a racemic mixture of valine was sublimated until the acid condensed, and formed a crystal-based conglomerate. Pure enantiomer crystals were then extracted, and the container heated further.
This lead to an increase in the initial enantiomeric excess, the group says. “One can imagine similar processes occurring near volcanoes where temperature gradients are enormous and such scenarios would have been plausible on primitive Earth,” the team leader says.
“It is true that gas-solid transformations may not be as general as solid-liquid equilibria, but you never know what is going to turn out to be useful,” Viedma adds.
“This is an interesting project in terms of the intriguing conundrum of the origin of biological chirality as well as extensions to the manufacture of chiral substances,” he goes on to say.
“I have no doubt that it will change my understanding of how crystals grow in a profound way. Understanding this behavior might expand the range of what we can control in chemistry and materials science,” adds Michael McBride.
The expert, who was not involved in the new research, is an Yale University expert in chemical reactivity and physical properties of organic solids studies.
 14 DAY TRIAL //
14 DAY TRIAL //