A team of Australian and British researchers, discovered that within our bodies, there is a protein called perforin, which punches holes in rogue cells, and kills them.
The researchers have deciphered the structure of the perforin pore, and as project leader Prof James Whisstock from Monash University says, “perforin is our body's weapon of cleansing and death.”
The principle is not complicated: when the immune system has detected a cancerous or virus-infected cells within the bloodstream, it activates these killer cells that basically execute it by lethal injection.
Professor Whisstock explains that the protein “breaks into cells that have been hijacked by viruses or turned into cancer cells and allows toxic enzymes in, to destroy the cell from within.
“Without it our immune system can't destroy these cells, [and] now [that] we know how it works, we can start to fine tune it to fight cancer, malaria and diabetes,” he adds.
The destruction mechanism of the infected cells is simple: when the killer cells get in close contact with the target, they release the perforin protein into a narrow space between the cells.
The protein will punch holes in the membrane of the infected cell and allow destructive enzymes to get in, destroying it.
The first time that someone observed that the human immune system could punch holes in target cells was 110 years ago, and that someone - Jules Bordet, won the Nobel Prize.
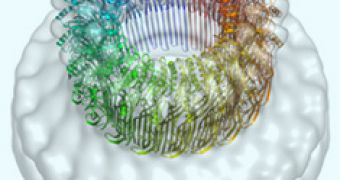
But it wasn't until now, that the structure of perforin – both in the water-soluble state and in the membrane pore, was explained.
Researcher Helen Saibil of Birkbeck College London, said that “the first major step in understanding perforin came from the discovery that it is related to a family of bacterial toxins, including pneumolysin, which we had previously studied.
“We went on to obtain an electron microscopy map of perforin pores, and we could see some similarities to pneumolysin pores but we couldn't interpret the structure in detail.”
Every pore contains 19 to 24 molecules of the protein, and can make an entrance of 130-200 Angstroms in width, and this discovery was made possible by the Australian Synchrotron, and the powerful electron microscopes at Birkbeck, the Royal Society of Chemistry reports.
Apparently, the main parts of the perforin molecule are very similar to those from toxins developed from bacteria like anthrax, listeria and streptococcus.
Prof Joe Trapani, head of the Cancer Immunology Program at Peter MacCallum Cancer Center in Melbourne said that “the molecular structure has survived for close to two billion years, we think.”
“This work is a dramatic illustration of the importance of the synchrotron,” added Whisstock.
“We simply couldn't have done it without this wonderful facility.”
Hagan Bayley of the University of Oxford, UK said that “this is a wonderful extension of the breakthrough work of 2007.
“The weapons used by our immune cells and by pathogenic bacteria have a common origin, but now it seems they swing their swords in different ways.”
Perforin is a very powerful molecule, and when it's not working properly, the body can't fight infected cells.
The molecule is also the culprit for autoimmune disease conditions, or for tissue rejection after a bone marrow transplant.
The team of researchers has received a $1 million grant from the Wellcome Trust, which will allow them to work on boosting perforin for more effective cancer protection and therapy for severe diseases such as cerebral malaria.
The discovery of this killer mechanism is published in the science journal Nature.
 14 DAY TRIAL //
14 DAY TRIAL //