Scientists have suspected that proteins inside the human body vibrate at different patterns for a long time, but thus far studies conducted to confirm this hypothesis have yielded inconclusive results. A new investigation by researchers at the University at Buffalo and Hauptman-Woodward Medical Research Institute provides the first real evidences that this is indeed the case.
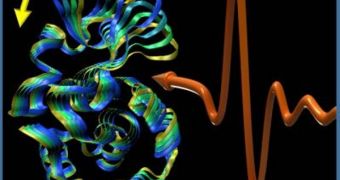
The discovery was made possible through the use of an imaging technique called terahertz near-field microscopy, which enabled researchers to use terahertz radiation to probe individual proteins. In response to these wavelengths, antibacterial proteins called lysozymes wiggles, the team reveals.
Another interesting discovery was the fact that the vibrational effect persisted in these molecules for a much longer time than predicted. Previous theoretical approaches had suggested that any vibrations exhibited by proteins would dissipate quickly, but this was not what investigators saw.
Study leader Andrea Markelz, PhD, a professor of physics at the University at Buffalo, says that the tail of the vibrational effect the team observed resembled the ringing of a bell. One of the potential explanations for why proteins vibrate is that this ability enables them to quickly change shape.
The quicker they change shape, the quicker they are able to bind to other proteins, a skill that is essential for animal life. Without protein binding, DNA replication, cellular repair processes and absorbing oxygen from the bloodstream become impossible, EurekAlert reports.
“People have been trying to measure these vibrations in proteins for many, many years, since the 1960s. In the past, to look at these large-scale, correlated motions in proteins was a challenge that required extremely dry and cold environments and expensive facilities,” Markelz explains.
“Our technique is easier and much faster. You don't need to cool the proteins to below freezing or use a synchrotron light source or a nuclear reactor – all things people have used previously to try and examine these vibrations,” she adds.
Details of the research appear in a paper published in the January 16 issue of the top scientific journal Nature Communications. The team says that the technique used in the new study may soon be applied to study the influences of artificial and natural protein inhibitors.
These molecules are able to prevent proteins from carrying out their roles in the body, by blocking their vibrations at different frequencies. By understanding this mechanism, scientists may soon be able to develop new therapies that either block or promote the actions of protein inhibitors.
“The cellular system is just amazing. You can think of a cell as a little machine that does lots of different things – it senses, it makes more of itself, it reads and replicates DNA, and for all of these things to occur, proteins have to vibrate and interact with one another,” the team leader concludes.
 14 DAY TRIAL //
14 DAY TRIAL //