An international team of researchers was recently able to figure out why proteins such as myoglobin are able to carry out their functions even in the absence of water. The study will contribute to the development of advanced wound dressing and next-generation biochemical gas sensors, among others.
Without proteins performing their vital biological functions, lifeforms would not survive. Some time ago, researchers noticed that the tiny molecules were able to survive and operate in dry environments, even though water was believed to be indispensable to their functioning.
The team that carried out the study featured experts from the University of Bristol (UB), the Institut de Biologie Structurale (IBS) in Grenoble, the Australian National University, the Institut Laue Langevin and the Jülich Centre for Neutron Science.
Details of the research effort were published in the latest issue of the esteemed scientific Journal of the American Chemical Society. Completing this study was only made possible via the use of a state-of-the-art neutron scattering technique, the group explains.
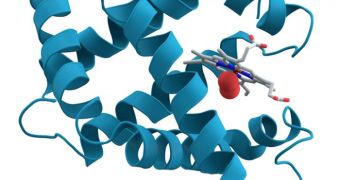
The work focused on the protein myoglobin, which is located in the muscle tissue of all vertebrates, and whose role is to bind oxygen. Using the aforementioned research method, the group was able to determine that myoglobin can be enclosed in a sheath of surfactant molecules.
When this happens, the protein behaves just as if it was surrounded by water. This discovery opens the way for using these essential molecules for a variety of applications that were previously inaccessible to researchers.
An interesting aspect of the research was that the surfactant molecules coating myoglobin were entirely synthetic. The work follows on the path opened by a 2010 UB study, which demonstrated that proteins can function even in environments with severely reduced amounts of water available.
“These discoveries have increased our fundamental understanding of proteins and how they behave, which could create many new opportunities for their application in industrial processing and in medical technologies,” UB School of Chemistry expert, Dr. Adam Perriman, explains.
“The fact that our proteins can happily perform their function outside of water, a substance generally thought to be vital for life, really drives home just how robust these biological nanomachines are,” he concludes.
 14 DAY TRIAL //
14 DAY TRIAL //