A group of investigators at the Massachusetts Institute of Technology (MIT) announces the creation of a new method for determining the form and function of proteins at extremely brief intervals. This approach is significantly more efficient and useful than anything currently available for researchers.
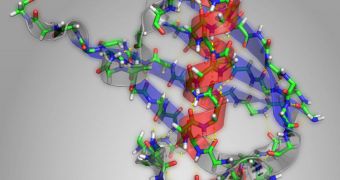
One of the things hampering the study of proteins is the fact that they usually require some degree of treatment before they are put under the microscope. This usually involves crystallizing the molecules, and then using an observations method called X-ray protein crystallography to study their structure.
However, the shape of proteins is oftentimes what dictates their function, and their shapes change constantly. By crystallizing the molecules, researchers lose access to important data, such as how the proteins change over time, and how their function changes to follow suit.
With the new technique developed at MIT, experts can now watch how proteins change their shape live, at extremely brief intervals, of just a few picoseconds, or trillionths of a second. The best part is that the molecules require no treatment beforehand.
The research was led by MIT chemistry professor Andrei Tokmakoff and postdoctoral researcher Carlos Baiz. The team published details of its approach in the March issue of the journal Analyst.
The new observations technique was developed from two-dimensional infrared spectroscopy, an approach in which molecules are bombarded with pulses of IR light, and the resulting vibrations are then analyzed.
During the new study, the scientists were able to create a new method for handling incoming data, which also enables the correlation of additional information, such as those covering the common structural elements of proteins. This combination reveals a wealth of more details.
Proteins have the tendency to fold themselves into secondary structures just after they are first assembled. These arrangements are called alpha helices and beta pleated sheets. In this study, the MIT group analyzed how bonds between carbon and oxygen in molecules changed under IR light.
“In principle, the full structure of the protein is represented in the spectrum. The trick is how to get out the information,” explains Baiz, who was also the lead author of the research paper.
“This is the first method that will allow us to take snapshots of the structure of the protein as it’s denatured. Usually the way people look at proteins is they start with the unfolded state and they end up with the folded state, so you have two static structures,” he adds.
“What we can do now is look at all the structures along the pathway,” Baiz concludes.
 14 DAY TRIAL //
14 DAY TRIAL //