All industrialized countries in the world, without any exceptions, have a very strong chemical industry, which uses many substances for a variety of reactions. Catalysts are among the most widely used, and some reactions need specific chemicals to make them work. Some of these chemicals may be gold or platinum, which makes the entire production process very expensive. Now, experts propose a new approach to Mendeleev's Periodic Table of Chemical Elements, which could see many chemicals being replaced with others that “look” and act like them, but are actually made up of other, cheaper elements.
Alchemists have tried for centuries to convert lead into gold, but to little avail. However, it would now appear that a new type of alchemy is actually possible. Scientists at the Pennsylvania State University (Penn State) now propose that various mash-ups of chemical elements could be arranged in such a manner that they would act like a single catalyst, or reactant, in a chemical reaction, replacing some of the most expensive materials currently in use. In addition, the new class of substances could also be used to remove polluting chemicals from the industry, the group says, quoted by PhysOrg.
The new work was conducted specifically on certain atoms, which, when combined, exhibited the same electronic signature as another chemical element. “The findings could lead to much cheaper materials for widespread applications such as new sources of energy, methods of pollution abatement, and catalysts on which industrial nations depend heavily for chemical processing. We're getting a whole new perspective of the periodic table,” the leader of the research team, expert A. Welford Castleman Jr., explains. He is also the Eberly Distinguished Chair in Science and Evan Pugh Professor at the Penn State Departments of Chemistry and Physics.
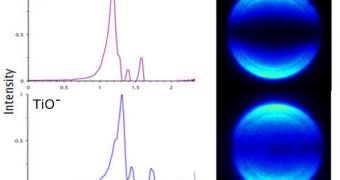
The team used a technique known as photoelectron imaging spectroscopy to analyze the similarities and differences between various pairs of chemical elements, including titanium monoxide and nickel, zirconium monoxide and palladium, and tungsten carbide and platinum. “The method allows us to determine the binding energies of the electrons and also to observe directly the nature of the orbitals in which the electrons resided before they were detached. We found that the amount of energy required to remove electrons from a titanium-monoxide molecule is the same as the amount of energy required to remove electrons from a nickel atom,” Castleman says.
“The same is true for the systems zirconium monoxide and palladium and tungsten carbide and platinum. The key is that all of the pairs are composed of isoelectronic species, which are atoms with the same electron configuration,” he concludes.
 14 DAY TRIAL //
14 DAY TRIAL //