A new method of producing nanoscale wires could lead to the development of more advanced sensors for detecting dangerous chemicals, such as toxic gases. In order to produce these devices, researchers first need to be able to produce gold nanowires.
This has proven to be quite difficult, but recently a team of experts at the University of Pittsburgh was able to solve this issue. They created a new type of scaffold, which can be used to coax gold into forming nanowires for multiple application.
The investigation was led by principal investigator Alexander Star, who holds an appointment as an associate professor of chemistry with the Kenneth P. Dietrich School of Arts and Sciences at Pitt.
The research team collaborated with colleagues at the National Energy Technology Laboratory (NETL). Details of the study were published in the January 22 issue of the esteemed Journal of the American Chemical Society (JACS).
In the paper, entitled “Welding of Gold Nanoparticles on Graphitic Templates for Chemical Sensing,” the team explains how the new scaffolds are used for gold nanowire self-assembly. The technique is as simple as it is ingenious.
“The most common methods to sense gases require bulky and expensive equipment. Chip-based sensors that rely on nanomaterials for detection would be less expensive and more portable as workers could wear them to monitor poisonous gases, such as hydrogen sulfide,” Star says.
Gold nanowires were identified as one of the cheapest, most effective tools capable of detecting hydrogen sulfide. The reason for that is that gold has a tremendous affinity for sulfur. These natural traits are augmented by the intrinsic properties of nanomaterials, the team adds.
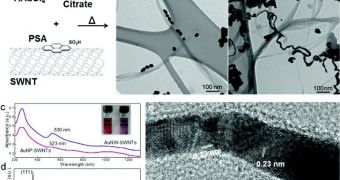
During the experiments the team conducted, carbon nanotubes and graphene played an important role in creating the gold nanowires. X-ray diffraction and transmission electron microscopy were used to image the tiny structures, which were first modeled in computer simulations.
“To produce the gold nanowires, we suspended nanotubes in water with gold-containing chloroauric acid. As we stirred and heated the mixture, the gold reduced and formed nanoparticles on the outer walls of the tubes,” Star explains.
“The result was a highly conductive jumble of gold nanowires and carbon nanotubes,” the investigator concludes.
 14 DAY TRIAL //
14 DAY TRIAL //