The combination pill Stribild was approved by the US Food and Drug Administration (FDA) yesterday, August 27, for treating HIV-infected patients who received no previous treatments. The drug features two well-known compounds, as well as two new ones.
The primary components of Stribild are elvitegravir, cobicistat, emtricitabine and tenofovir disoproxil fumarate. Destined for adult HIV patients, the new medication is taken only once a day, and requires no other supplements.
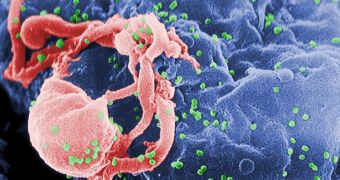
Scientists have been working towards creating a single pill for HIV treatment for many years, but thus far to no avail. Until now, patients had to take several drugs daily, each of which addressed a specific component of the human immunodeficiency virus (HIV).
Now, all of their therapeutic actions are combined in a single pill, making it easier and cheaper for adults to get the treatment they need. The two newly-approved compounds in Stribild are elvitegravir and cobicistat.
The former acts by interfering with one of the key enzymes HIV uses to multiply inside the human body. Microbiologists classify it as an HIV integrase strand transfer inhibitor. What it does is prevent the virus from creating more copies of itself.
The second compounds, cobicistat, is a pharmokinetic enhancerm, does not target the virus directly. Rather, it inhibits the actions of an enzyme responsible for metabolizing key compounds in some HIV drugs. Its main role in Stribild is to prolong the effects of elvitegravir.
As for the other two compounds, emtricitabine and tenofovir disoproxil fumarate, the chemicals have been used since 2004, under the brand name Truvada. What they do is block another key enzyme HIV needs for multiplying.
“Through continued research and drug development, treatment for those infected with HIV has evolved from multi-pill regimens to single-pill regimens. New combination HIV drugs like Stribild help simplify treatment regimens,” says Edward Cox, MD, MPH.
The official holds an appointment as the director of the Office of Antimicrobial Products, at the FDA Center for Drug Evaluation and Research.
“Results showed between 88 percent and 90 percent of patients treated with Stribild had an undetectable amount of HIV in their blood [at 48 weeks of treatment], compared with 84 percent treated with Atripla and 87 percent treated with Truvada plus atazanavir and ritonavir,” the FDA says.
 14 DAY TRIAL //
14 DAY TRIAL //