Ever since the field of nanotechnology began developing, researchers have recognized the importance that these small particles had in fighting conditions such as cancer. Destroying tumors by using such an approach is possible, but a lot of care needs to be exercised in order to ensure that as little healthy tissue as possible is affected. Now, an international collaboration of scientists investigates a new type of nanomachinem that release drugs directly inside living cancer cells.
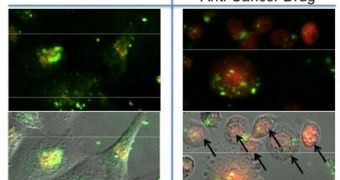
The researchers are based at the Yonsei University, in South Korea, and the California NanoSystems Institute, which is based at the University of California in Los Angeles (UCLA). The nanomachines the group is looking into are innovative because they are only activated when a remote, oscillating magnetic field is applied. The drug-carrying structures are in fact porous nanomaterials driven by a magnetic core, the researchers say, and these machines represent the first time materials of this class have been used for such applications.
The new particles also hold promise for producing more advanced contrast agents, to be used in imaging techniques such as MRI. “The hydrophobic [water-repellent] nature of the interior of the pores, as well as the ability to functionalize the silica surface with hydrophilic [water-loving] functionalities, makes these particles attractive for anti-cancer drug delivery. Adding a magnetic core to the silica-based nanoparticles is of interest for its potential applications in magnetic resonance imaging, as addition of the magnetic core may make it useful as a contrast agent,” says UCLA professor of chemistry and biochemistry Jeffrey Zink. In a research paper accompanying the findings, which appears in the July issue of the respected Journal of the American Chemical Society, the team argues that the new method could be used to target sick, tumorous cells only. This would reduce the amount of healthy tissue destroyed as side-effect to nearly zero, which could help contribute the healing process. “The novel magnetic-core silica nanoparticles are effective in activating nanovalves which release anti-cancer drugs when they are exposed to an oscillating magnetic field,” Zink explains.
The new work was conducted with funds secured from the UC Toxic Substances Training and Research Program, the US National Science Foundation (NSF), the NanoMedical National Core Research Center, and the Creative Research Initiative Program of Korea. The California NanoSystems Institute at UCLA is an integrated research center operating jointly at UCLA and UC Santa Barbara whose mission is to foster interdisciplinary collaborations for discoveries in nanosystems and nanotechnology; train the next generation of scientists, educators and technology leaders; and facilitate partnerships with industry, fueling economic development and the social well-being of California, the United States and the world.
 14 DAY TRIAL //
14 DAY TRIAL //