Nanoelectronic designers at the Rice University, in the United States, announce the development of a new technique for producing alloy materials containing the critical 2D carbon compound graphene.
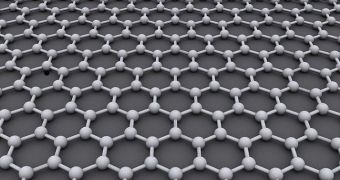
This material has a hexagonal, honeycomb-like structure featuring carbon atoms exclusively. It features a host of chemical and physical properties that were never before observed in other substances. Graphene was first synthesized in 2004.
Already, the University of Manchester team that discovered it was awarded the 2010 Nobel Prize in Physics. At this point, physicists want to use it primarily as a replacement for silicon in electronic equipment and circuits.
But doing so implies doping graphene with other nanomaterials that are not conductive to electrical current. In fact, experts need to develop a way of applying the non-conductive substances in an intricate pattern over sheets of graphene.
What the Rice University did was find a way of doing just that. Experts here used one-atom-thick sheets of boron and nitrogen for the job. This material is known among experts as white graphene, because it has the same physical structure as the carbon compound.
According to a paper published in the scientific journal Nano Letters, the Rice investigators were able to control the electronic properties of graphene/white graphene alloys using established chemical methods, therefore further improving on the possible applications this technique could have.
“We found there was a direct relationship between the useful properties of the final product and the chemical conditions that exist while it is being made,” Rice materials scientist Boris Yakobson says.
“If more boron is available during chemical synthesis, that leads to alloys with a certain type of geometric arrangement of atoms,” says the expert, the lead author of the study and of the research team.
“The beauty of the finding is that we can precisely predict the electronic properties of the final product based solely upon the conditions – technically speaking, the so-called 'chemical potential' – during synthesis,” he goes on to say,.
The new study was made possible by funds secured from the Department of Energy (DOE) and the Office of Naval Research. Computational resources were supported by the National Institute for Computational Sciences and the National Science Foundation (NSF).
This week alone, experts at Rice presented another two papers on graphene, both of which are innovative approaches to producing the material in bulk. This third paper indicates that the university is indeed shaping up to be a leader in this field of research.
 14 DAY TRIAL //
14 DAY TRIAL //