Crystallization is the name given to the process that sees the transformation of a solution, melt or gas into solid crystals, through precipitation. The phenomenon takes place relatively slow when compared to other types of chemical reactions, and scientists have recently discovered one of the reasons for that. It would appear that the angles present in the vessels used during crystallization are a very important factor, ScienceNow reports.
In a paper published in the latest issue of the respected Journal of the American Chemical Society, experts at the University of Surrey, in the United Kingdom, explain that the process can take place at up to 48 orders of magnitude faster at angles of about 70 degrees, as opposed to forming on flat surfaces. The main reason why crystal formation is usually slow is the fact that the arrangements have a very specific and precise internal structure, which cannot be achieved during fast chemical reactions.
The trick is to get the first molecules to self-organize, a process known as nucleation. As this happens, the first particles to crystallize basically turn into a template for those around them, facilitating the entire process. In the standard crystallization process – the one that is taught in college chemistry classes – teachers ask students to scratch the inside of a flask, telling them that this would increase the formation rate of crystals.
The reason why this is accurate is the fact that grooves and pits in glass surfaces in essence help crystals grow. They act as nucleation hot spots. More of these hot spots mean that nucleation begins in more places at the same time, and therefore influence a lot more molecules around these places than if they only started at a single location. University of Surrey physical chemists Amanda Page and Richard Sear wanted to learn exactly how grooves help nucleation.
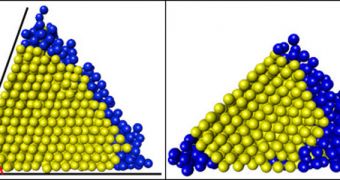
The way the did that was to run computer simulation on hypothetical scenarios, such as what would happen to droplets of simple molecules – such as methane and argon – when placed inside V-shaped grooves. They then ran comparative tests, changing the angle of the groove, and observing how long it took for nucleation to occur. In the online paper, they report that the best and fastest results were obtained when the angle was precisely 70 degrees.
However, they say that the angle is entirely dependent on the substances being used for crystal production. The angle works best for methane and argon because they like to form into a type of pattern called a face-centered cubic lattice, which is compatible with a 70-degree angle. Smaller angles, like a 45-degree one, proved to hinder the nucleation process. “The crystal says, 'I want to be 70 degrees,' and the wedge says, 'No, you have to be 45 degrees.' So there's frustration,” Sears explains.
 14 DAY TRIAL //
14 DAY TRIAL //