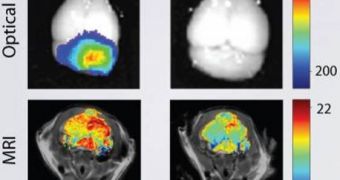
Brain tumors are notoriously lethal and fast-evolving, and therefore intervening with surgery is a top priority in most medical cases. In the OR, doctors rely heavily on MRI scans and optical imaging of the affected area for their procedure, and it is therefore essential that they have the best possible data, so that as much as possible of the tumor is extracted. Now, scientists at the University of Washington have developed a new viewing method, which makes use of nanoparticles to better evidence tumors.
Each human brain is protected very effectively from infections in the blood via the blood-brain barrier, a layer of cells that simply does not allow for pathogens to move through, except in very specific conditions. The nanoparticles devised at UW have the ability to penetrate this barrier without inflicting any damage to it, and then affix themselves to the cancerous tissue. Their fluorescence offers the perfect substrate for an MRI. Images obtained using nanoparticles proved to have much better contrasts and borders, making it easier for surgeons to orient themselves in the operating room.
“Brain cancers are very invasive, different from the other cancers. They will invade the surrounding tissue and there is no clear boundary between the tumor tissue and the normal brain tissue,” UW Professor of Materials Science and Engineering Miqin Zhang says. The expert is also the lead author of a new study detailing the finds, published in this week's issue of the respected journal Cancer Research. The work was funded by the National Institutes of Health (NIH), the Jordyn Dukelow Memorial Fund and the Seattle Children's Hospital Brain Tumor Research Endowment.
“If we can inject these nanoparticles with infrared dye, they will increase the contrast between the tumor tissue and the normal tissue. So during the surgery, the surgeons can see the boundary more precisely. We call it 'brain tumor illumination or brain tumor painting.' The tumor will light up,” the scientist adds. Having the ability to accurately distinguish between healthy and affected tissue is extremely relevant for surgeons. Nowadays, cognitive impairments are notable side-effects of brain surgeries, as some doctors involuntarily cut out some healthy tissue in addition to the tumorous one, because the boundary is not very clearly shown on images and the MRI.
The active ingredient that is responsible for the fluorescent nanoparticles traveling to the cancerous cells alone, and nowhere else, is called chlorotoxin, and it is a compound derived from scorpion venom. The substance has been under investigation for quite some time now, as it exhibits outstanding tumor-seeking properties. The particles used in the experiments are only 33 nanometers in diameter, which is about three times less than the average size of other nanoparticles, used in tumors and organs in other parts of the body.
“Precise imaging of brain tumors is phenomenally important. We know that patient survival for brain tumors is directly related to the amount of tumor that you can resect. This is the next generation of cancer imaging. The last generation was CT, this generation was MRI, and this is the next generation of advances,” UW School of Medicine Professor and Chair of Neurological Surgery Richard Ellenbogen, also a co-author of the PNAS paper, concludes.
 14 DAY TRIAL //
14 DAY TRIAL //