One of the main dangers plaguing all sorts of medical tools, devices and human implants at this point comes from a bacterium known as Staphylococcus epidermidis. Opportunistic by nature, the organism regularly lives on our skins, and is as harmless as it comes. However, when it hitches rides inside us via needles, implants and other medical instruments, it can cause severe infections. The bad thing about this is that it acts just like MRSA – mostly in hospitals, and on people with already weak immune systems. Now, experts at the Brown University have finally found a match for it.
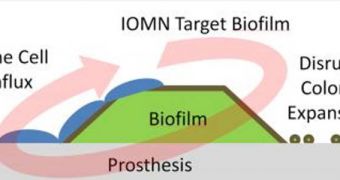
Whenever it gets inside the human body, this bacterium has the nasty habit of lodging itself on the implants, and then conglomerating in colonies. To shield themselves from adversaries, the bacteria secrete a protective layer of blocking substances, called a biofilm. This material is so strong, that all the cells in the immune system bang their head against it to little avail. The only solution to this problem is removing the implant, because otherwise the colony just keeps growing and growing.
According to statistics published in the latest issue of the scientific journal Clinical Infectious Diseases, about 2.5 percent of all hip and knee implant patients in the United States get infected with Staphylococcus epidermidis each year, sometimes to fatal outcomes. For all these thousands of people, experts Thomas Webster, a Brown University biomedical engineer, and graduate student Erik Taylor have developed a new, nano-sized “killer” particle, able to search and destroy the bacterial biofilm.
The researchers, who published the details of their innovative material in the latest issue of the International Journal of Nanomedicine, say that the new iron oxide complex has the ability to zero in on the location of the implant, penetrate the bacterial defensive layers, and directly kill the microorganisms. In their experiments, the two observed that nearly 28 percent of bacterial colonies had been removed from an implanted prosthetic device, within 48 hours of injecting just ten micrograms of the nanoparticle “killer.”
The two explain that the nanoparticles managed to penetrate the biofilm not through chemical reaction, but through sheer, magnetic horsepower. Because iron oxide is highly magnetic, they used a magnet placed directly under the implant to essentially draw the nanoparticles (with an average diameter of eight nanometers) straight through the shield. Once inside the colonies, they started destroying the bacteria one by one. “There will be a continual killing of the bacteria until the film is gone,” Webster says.
 14 DAY TRIAL //
14 DAY TRIAL //