According to investigators at Caltech and JPL, it would appear that existing air-quality model are distorting the reality as far as atmospheric ozone concentrations go.
The team explains that the existing simulations may in fact be downplaying the significance that these chemicals are having in the atmosphere, and adds that its developed a method that could lead to more accurate estimates of ozone levels.
What the research team did was essentially analyze in great detail the key chemical reactions that have been linked to the formation of pollutants in various types of air, especially in smoggy one.
With the new chemical characterizations in, the study team showed that, in certain areas of Los Angeles, existing ozone models underestimate the chemical's concentrations by 5 to 10 percent.
The work was conducted by investigators at the California Institute of Technology (Caltech) and the NASA Jet Propulsion Laboratory (JPL), which are both located in Pasadena.
According to professor and team member Mitchio Okumura, the new discoveries will most likely carry “a small but significant impact on the predictions of computer models used to assess air quality, regulate emissions, and estimate the health impact of air pollution.”
The expert is one of the principal investigators for this research, and also a Caltech professor of chemical physics. He is also one of the authors for a new paper detailing the findings, which appears in the latest issue of the top journal Science.
“This work demonstrates how important accurate laboratory measurements are to our understanding of the atmosphere,” explains the leader of the research effort. JPL senior scientist Stanley P. Sander.
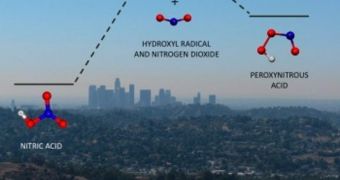
One of the most important findings in the new study was how ozone is formed from chemical reactions between nitrogen dioxide (NO) and the hydroxyl radical (OH), in the presence of sunlight and volatile organic compounds (VOC).
For years, chemists have known that this reaction produces nitric acid (HONO2), a molecule that dissolves in rainwater, and which gets removed from the atmosphere.
“It's basically a sink for these radicals, taking them out of the ozone equation and thus slowing down the rate of ozone formation,” Okumura says.
But the team found what they suspected all along, a second type of reaction, that leads to the formation of peroxynitrous acid, or HOONO.
This compound is unstable, and breaks back down into HONO2, NO2 and OH, to fuel the ozone-producing cycle.
“Solving this atmospheric chemistry problem required us to use many tools from modern chemical physics,” the Caltech professor says.
“This work was the synthesis of two very different and difficult experiments. While neither experiment in isolation provided definitive results, by combining the two data sets, the parameters needed for air-quality models could be precisely determined,” adds Andrew Mollner.
He is the first author of the Science paper, and also a former Caltech graduate student. Mollner is now based at the Aerospace Corporation.
 14 DAY TRIAL //
14 DAY TRIAL //