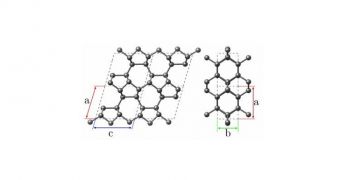
Experts from the University of Basel in Switzerland, led by Maximilian Amsler, predict the existence of a previously-unsuspected allotrope (form) of carbon. The new structure was hinted at in a computer simulation, which suggested that the material may appear during high pressure experiments.
Thus far, experts know that carbon can organize in diamonds, graphite, graphene (obtained for the first time in 2004) and fullerenes. The new allotrope can theoretically be obtained by compressing graphite under 10 gigaPascals of pressure, at room temperature, a paper published in arXiv indicates.
Properties such as resistivity, optical transmittance and reflectance are expected to change dramatically in this arrangement. The model shows that carbon may even undergo a phase chance under such conditions. The team calls the proposed form M10-carbon.
All atoms in this allotrope would be linked together by sp3 bonds, which would make it as tough as diamond, but more stable than graphite at pressures exceeding 14 Gpa.
 14 DAY TRIAL //
14 DAY TRIAL //