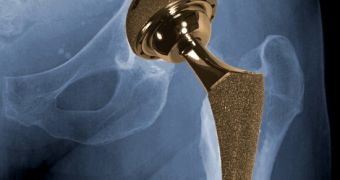
According to a new report made public by the US Food and Drug Administration (FDA), all-metal hip implants need be linked to both soft tissue damage and pain.
Because of this, it is likely that an individual who was fitted with one such metal-on-mental hip implant will at one point require additional surgeries in order to have their implant removed and replaced with a new one.
Despite the fact that all-metal hip implants are supposedly more sturdy than other options patients have at their disposal, it looks like the disadvantages might sometimes outweigh the benefits.
Thus, the FDA claims that these implants can lose metal at points where two components connect. This translates into the implant itself becoming prone to failure, and into bone and soft tissue damage.
“Metal release will cause some tiny metal particles to wear off of the device into the space around the implant,” reads the official FDA website.
Furthermore, “Wear and corrosion at the connection between the metal ball and taper of the stem may also occur. Some of the metal ions (e.g. cobalt and chromium) from the metal implant or from the metal particles will enter the bloodstream.”
For the time being, the FDA is unable to say how each patient will respond to his/her being fitted with one such metal-on-mental hip implant.
As well as this, they lack information concerning the ways in which various concentration levels of metal pieces lost in a patient's body or bloodstream will affect their health, causing additional health problems, and what those health problems would be.
“Different people will react to these metal particles in different ways. At this time, it is not possible to predict who will experience a reaction, what type of reaction they might have, when the reaction will occur, or how severe the reaction will be,” the FDA says.
Therefore, they advise that each patient who is looking into the possibility of having his/her hip fitted with a metal implant take the time to discuss both the risks and the benefits with their surgeon.
The FDA also recommends that people fitted with all-metal hip implants who do not display any adverse effects (i.e. skin rash, cardiomyopathy, auditory, or visual impairments, psychological status change, renal function impairment, thyroid dysfunction) still present for follow-up consultations at least one every one-two years.
 14 DAY TRIAL //
14 DAY TRIAL //