Stem cells have been one of the most promising branches of bioengineering ever since the first ones were harvested, and now we have a strong piece of proof that the funds funneled into their research were well spent.
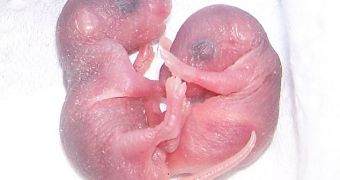
To get right to the point, a group of Japanese researchers from Kyoto University have succeeded in creating viable eggs from stem cells. Once done, they transplanted them into female mice and waited to see if the gestation proceeded properly.
In the first part of the process, the scientists took embryonic stem cells and induced pluripotent stem cells (iPSCs) from them.
Those they subsequently transformed, slowly, into egg precursors by means of a host of signaling molecules. After applying lab-grown ovary tissue, the cells matured into eggs over a period of four weeks.
It was those eggs that were transplanted into foster mothers, which later produced healthy and, equally importantly, completely fertile pups.
“It was always believed that making sperm was probably easier,” says Davor Solter, a developmental biologist at the Institute of Medical Biology in Singapore. He was not involved with the study, but is familiar with it. “The oocyte is the thing which makes the whole of development possible.”
Proving Solter's point is the fact that the Kyoto team succeeded in creating sperm cells before. Those are much smaller and simpler though, while ovums are considered “giants” among cells.
They are also far more complex, compared to all other cell types, not just male reproductive cells, due to how they divide. As opposed to mitosis (both sets of chromosomes are copied), ovums, or oocytes, perform meiosis (only one copy of each chromosome stays in each cell).
The most significant part about the breakthrough is just that: the process works. In other words, it proves once and for all that stem cell treatments can be used to treat infertility, assuming all the potential ethical issues are surmounted.
 14 DAY TRIAL //
14 DAY TRIAL //