A team of scientists with the Life Sciences Division at the US Department of Energy's (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab) announces the discovery of an interesting role played by a certain type of metabolism in cancer. The findings could lead to new therapies against several forms of this dreaded condition.
For many years, scientists have largely ignored the role of metabolic activities in cancer, but a team led by distinguished scientist Mina Bissell addressed this shortcoming in the new investigation. She is a leading global authority on breast cancer research.
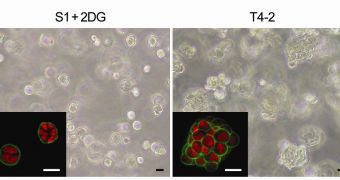
Her team was able to successfully demonstrate that glucose metabolism taking place in the presence of oxygen – a process called aerobic glycolysis – is in itself a cancerous event, rather than an effect of malignant cells performing damaging activities related to the advancement of cancer.
Bissell carried out the study with Japanese postdoctoral fellow Yasuhito Onodera, who is now an assistant professor in Japan. The duo looked carefully at how glucose transporter proteins are expressed in human breast cells. The molecule GLUT3 was the main target of the research.
“A dramatic increase in sugar uptake could be a cause of oncogenesis. Furthermore, through a series of painstaking analysis, we have discovered two new pathways through which increased uptake of glucose could itself activate other oncogenic pathways,” Bissell explains.
“This discovery provides possible new targets for diagnosis and therapeutics,” she goes on to say.
In a paper published in a recent issue of the Journal of Clinical Investigation (JCI), the team showed that GLUT3 concentrations are roughly 400 times higher in malignant cells, as opposed to their healthy counterparts. This was demonstrated to lead directly to cancer growth.
“We found that overexpression of GLUT3 in the non-malignant human breast cells activated known oncogenic signaling pathways and led to the loss of tissue polarity and the onset of cancerous growth,” the team leader explains.
“Conversely, the reduction of GLUT3 in the malignant cells led to a phenotypic reversion, in which the oncogenic signaling pathways were suppressed and the cells behaved as if they were non-malignant even though they still contained the malignant genome,” she concludes.
 14 DAY TRIAL //
14 DAY TRIAL //