The human body is, indeed, one of the most mysterious and best put together constructs in the world, but its amazing complexity and functions must not lead people to believe that it is the work of a higher power. For example, Intelligent Design (ID) proponents have said for a long time that one of their main arguments against evolution is the fact that the basic components inside cells are so complex, that they could not have appeared by accident. In this line of reasoning, the mitochondria occupied the first position. Now, new studies show that its seemingly irreducible complexity is actually reducible.
The human cell is an amazing machine. Complex processes take place inside several times per second, and it's easy to see why someone would find it very easy to say that these structures couldn't have possibly appeared naturally. ID proponents argue, for instance, that there are cellular components that cannot be broken down into their smaller, functional components. They argue that this “simple complexity” clearly shows that a designer, a higher power, made them the way they are.
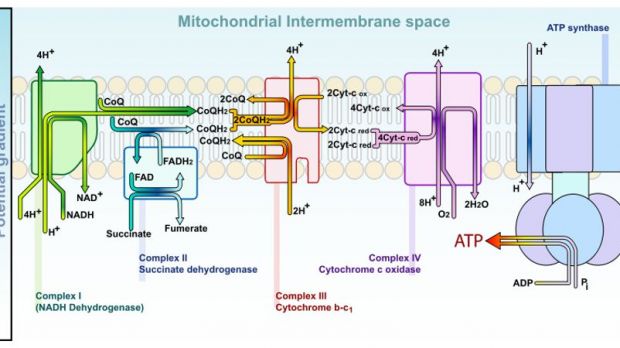
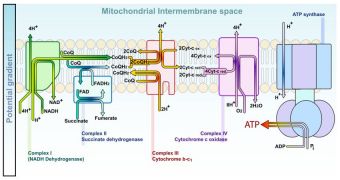
Mitochondria are often cited as an example. They are the cell's power plants, synthesizing the essential ATP (adenosine-triphosphate) nucleotide, without which nothing in the body can function. Pro-ID scientists have argued that the complexity and function of this cellular organelle couldn't have occurred naturally, as it is simply too efficient. But Yale University experts, led by postdoctoral cell biologist Sebastian Poggio, have now shown that the basic building blocks for mitochondria have been around long before complex microorganisms started appearing.
In a paper published in the Monday (August 24th) issue of the journal Proceedings of the National Academy of Sciences (PNAS), the team shows that the organelles appeared in free-living bacteria several billion years ago, long before more complex ones. With time, the complex organisms adapted to consuming the simple ones. Mitochondria predecessors thus became an integrated part of the complex microorganisms, and evolved with them over hundreds of millions of years. In time, developed mitochondria became essential to the cell's basic functions.
But the organelles could only have endured with the help of a protein mechanism known as TIM23, which bacteria do not, and did not produce. So, where did these proteins come from? ID proponents have relied on this question for some time, but the new study proposes a logical answer. The Yale experts say that it was a simple matter of reconversion and assigning new roles to already existing structures inside the cell. Mutations then also played an important part in establishing mitochondria as indispensable to the new types of cells.
One example to support this theory is the flagella, a structure in the cell that propels it forward in its environment. The basic building blocks of this “instrument” can be found elsewhere in the cell as well, but performing different tasks. “This analysis of protein transport provides a blueprint for the evolution of cellular machinery in general. The complexity of these machines is not irreducible,” Monash University molecular biologist Trevor Lithgow adds, quoted by Wired.
In their researches, the experts managed to discover two components of a prospective TIM23 machine inside bacterial cells. The third one, a transport mechanism, could have easily derived via basic mutation from a molecule called LivH, which is very common in proteobacteria. The build-up process is called “neutral evolution,” when new mechanisms that form do not provide the cells with either advantages or disadvantages. Once everything is in place, a simple mutation can trigger a chain of events that eventually leads to modern life.
“It hasn’t been possible up until this point to trace any of those proteins back to a bacterial ancestor. These three proteins don’t perform precisely the same function in proteobacteria, but with a simple mutation could be transformed into a simple protein transport machine that could start the whole thing off. You look at cellular machines and say, why on earth would biology do anything like this? It’s too bizarre. But when you think about it in a neutral evolutionary fashion, in which these machineries emerge before there’s a need for them, then it makes sense,” Dalhousie University cell biologist Michael Gray, who has not been part of the new research, concludes.
 14 DAY TRIAL //
14 DAY TRIAL //