Rensselaer Polytechnic Institute (RPI) scientists announce the development of a new type of lithium-ion battery, which is made up of the world's thinnest material, the carbon compound graphene.
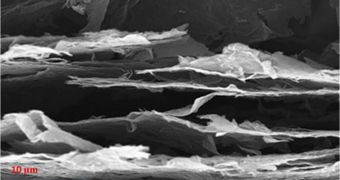
The team began the study by creating graphene paper. This material is basically a regular, one-atom-thick sheet of graphene, with no impurities or damages. All atoms making up this structure are arranged in a perfectly-hexagonal pattern.
Researchers then intentionally blemish the paper, zapping it with camera flashes or lasers. This results in the creation of imperfections such as cracks and pores, which are very important for the next step in the process to build high-density power storage devices.
These dents and cracks enable the graphene anode to be capable of charging and discharging up to 10 times faster than the conventional, graphite anodes used in lithium-ion batteries today. This could mean that the new generation of devices may be used to power electric cars.
The main problem of existing batteries is that they have limited power density. Additionally, they are unable to accept or discharge electricity very quickly, which leads to long battery recharging times.
These limitations are also one of the primary motivations behind installing a supercapacitor inside every electric car. Existing lithium-ion batteries are unable to support high-power functions, such as acceleration and breaking, all by themselves.
“Li-ion battery technology is magnificent, but truly hampered by its limited power density and its inability to quickly accept or discharge large amounts of energy,” explains RPI nanomaterials expert, Nikhil Koratkar.
“By using our defect-engineered graphene paper in the battery architecture, I think we can help overcome this limitation,” adds the expert, who also holds an appointment as the RPI John A. Clark and Edward T. Crossan professor of engineering.
“We believe this discovery is ripe for commercialization, and can make a significant impact on the development of new batteries and electrical systems for electric automobiles and portable electronics applications,” he goes on to say.
Details of how the new graphene-anode, lithium-ion batteries are made were published in a paper entitled “Photo-thermally reduced graphene as high power anodes for lithium ion batteries,” which appears in this week's issue of the esteemed scientific journal ACS Nano.
 14 DAY TRIAL //
14 DAY TRIAL //