Hydrogen bond exchange has been observed for the first time by a team of chemists from Kyoto University with the help of a scanning tunneling microscope while monitoring a single water dimer - two molecules of water bonded together. The hydrogen bond exchange takes place between two molecules with a frequency of a few tens of cycles per second, and the new observations may lead to a better understanding of the quantum tunneling and molecular vibrations involved in the exchange.
"The dynamics of hydrogen-bond exchange is visualized at single-molecule level in this work. Previous studies used spectral splittings as evidence for the exchange reaction. This direct observation enables us to clearly show that the exchange process involves quantum tunneling and also can be promoted by correlated vibrational excitation. This work is, I believe, of fundamental importance", says Hiroshi Okuyama from Kyoto University.
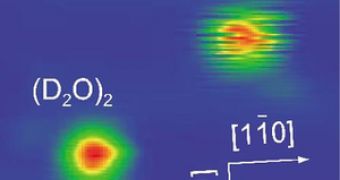
Water dimers are the simples systems containing hydrogen bonds, therefore they are the easiest to study. Water dimers are placed on a copper surface and chilled to a temperature of only 6 Kelvin. The dimers bonded to the copper surface through a hydrogen donor-acceptor exchange, while a heavy water dimer is placed in the vicinity of the sample for comparison.
Unlike the water dimer which presents hydrogen bond exchanges at a rate of 60 hertz, making it vibrate rapidly, the heavy water dimer appears stationary as its exchange rate is only of 1 Hz, or one cycle per second. This unique difference in molecule behavior is given by the hydrogen isotopes having different energy barriers, too large to be revealed through thermal processes.
Therefore the process can only be accounted for through quantum tunneling. The oxygen atoms in the water molecules are displaced in a way that the acceptor and donor molecules are brought to the same height, so that quantum tunneling can occur. The rapid exchange can be accounted for the fact that the molecules are being vibrationally exited.
Okuyama hopes that the study conducted by his team will bring better understanding of the applications that make use of such processes, like the catalytic reactions that take place on the electrode surfaces inside hydrogen fuel cells.
"The fuel cells mounted in future cars will reduce their exhaust gasses. The basic idea of the fuel cell is an electrochemical reaction at the electrode surfaces, where complex reactions proceed with the reactants and products including solvent water. At the microscopic level water molecules adsorb at the electrode surface, and dynamically form, break and exchange their hydrogen bonds with each other, probably promoting the electrode reactions", he said.
 14 DAY TRIAL //
14 DAY TRIAL //