A group of investigators at the University of Cambridge, in the United Kingdom, announces the development of a new strategy for creating nanoporous materials. They explain that this innovation makes it considerably easier to produce this class of substances, which have numerous applications.
For instance, they can be used to purify water or other liquids, as they retain impurities in their small pores. On the other hand, they can also be used to create extremely sensitive chemical sensors. But producing the materials themselves is a complex process.
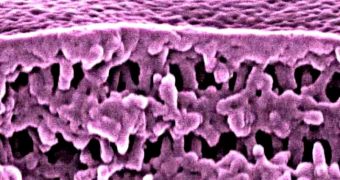
It has until now been believed that creating nanopores was only possible by using multiple materials. One of them was to be cleansed from the others through a chemical process, leaving behind very tiny holes. In order for that to happen, the pores had to be connected to themselves and to the outside of the material.
What the science team at Cambridge managed to create is a system for producing nanopores that does not require this contact to be made between the material that is to be removed from the original mix.
In a study published in the November 27 issue of the top scientific journal Nature Materials, experts detail their new method of producing nanopores in materials, called collective osmotic shock (COS).
As the name implies, this approach uses osmotic forces to carve out the pores. What is interesting about this method is that it can also create tiny hollows inside the target material even if the future pore is not connected to the outside, or to other pores.
“The experiment is rather similar to the classroom demonstration using a balloon containing salty water. How does one release the salt from the balloon? The answer is to put the balloon in a bath of fresh water,” explains Dr. Easan Sivaniah.
“The salt can’t leave the balloon but the water can enter, and it does so to reduce the saltiness in the balloon. As more water enters, the balloon swells, and eventually bursts, releasing the salt completely,” he goes on to say.
Sivaniah, who holds an appointment as a research scientist with the University of Cambridge Cavendish Laboratory, was the lead author of the new study. He explains that the new technique could lead to the creation of extremely sensitive sensors.
“In our experiments, we essentially show this works in materials with these trapped minor components, leading to a series of bursts that connect together and to the outside, releasing the trapped components and leaving an open porous material,” the investigator explains further.
Primarily, the team hopes to be able to adapt this technology for developing countries, where billions of people do not readily have access to potable water. Removing contaminants from this drinking water is absolutely essential for keeping those populations healthy.
 14 DAY TRIAL //
14 DAY TRIAL //