A fresh scientific investigation sheds light on the mechanisms employed by a genetic mutation in causing severe brain conditions, a research team announces. The finding is very important for developing new tools for detection, analysis and treatment of a common inherited neurodegenerative disease, the group writing a paper accompanying the discovery. The study appears in the March 24 issue of The Journal of Neuroscience.
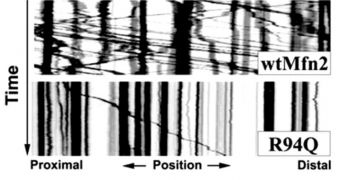
The disorder, called Charcot-Marie-Tooth (CMT), is caused by a protein that, apparently, is highly capable of disrupting the actions of the “power houses” of the cells. These are organelles known as mitochondria, whose primary role is to synthesize ATP (adenosine triphosphate), the main energy molecule that allows for life to exist. The way these mitochondria move alongside nerve fibers is essential for an efficient communication between brain and muscles, but the protein mutations are able to prevent that from happening.
The mitochondria therefore begin to move chaotically, preventing the accurate passing-on of signals from the cortex to the limbs. Responsible for this change is the protein mitofusin 2m, which has been determined in other studies to play an important part in the development of CMT. During the new experiments, researchers at the Washington University School of Medicine, led by expert Robert Baloh, MD, PhD, wanted to gain more insight into this phenomenon. The work was prompted by the fact that CMT affects around 2.6 million people annually.
The disease is largely characterized by loss of muscle tissue and sensation in the limbs, which occurs as brain-muscle communication channels are obstructed. “Our study provides the first evidence that mitofusins directly regulate the movement of mitochondria in nerve fibers. Furthermore, our work suggests the basis for this particular form of CMT is the abnormal movement of mitochondria in these fibers,” the team leader says. “This discovery places this type of CMT in the ever-growing list of neurodegenerative diseases caused by transport problems and strengthens the possibility of using general enhancers of this process as therapy for different types of diseases,” adds University of Antwerp expert Vincent Timmerman, PhD, who was not a part of the new investigation.
 14 DAY TRIAL //
14 DAY TRIAL //