A group of scientist at the University of California in Los Angeles (UCLA) announces a breakthrough in their understanding of how biological sensors function. These are critical components of cellular control systems, that is in charge of controlling the flow of potassium into the cell.
As such, these sensors are capable of triggering or blocking a host of cell activities, that involve this chemical. The particular type of sensor the team focused its study on is known as the “gating ring.”
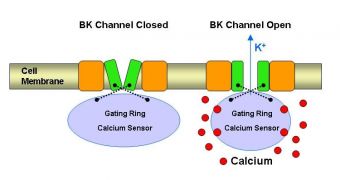
What it does is it allows potassium ions to pass through cellular walls or membranes, by opening up a dedicated channel under strict conditions. Potassium then goes on to regulate such critical processes as insulin secretion, brain signaling, and blood pressure.
Until now, the biophysical functioning of these sensors has remained somewhat elusive to researchers. But the UCLA team recently managed to strip the complex control systems of their natural mystery.
Now that the sensor's molecular mechanism have finally been revealed, experts can hope to start research aimed at exploring the new knowledge for the creation of new therapies against a wide array of medical conditions, including hypertension and genetic epilepsy.
Details of the new investigation and its conclusions will appear in the June 10 issue of the esteemed medical Journal of Biological Chemistry. The study has already been selected “Paper of the Week.”
The gating ring, the UCLA team explains, constitutes the intracellular part of the BK ionic channel, and is only activated when calcium ions (electrically-charged atoms) bound to it. If calcium is missing, then the potassium channels will remain sealed, and the chemical will be denied entry into the cell.
The opening of the BK channel, the research group found, is facilitated by the fact that the chemical energy of calcium is converted into mechanical work. This is why other chemicals cannot open this passageway, investigators believe.
“We were able to resolve the biophysical changes occurring in the sensor, under conditions resembling those present inside a living cell, so we believe that these transformations reflect the molecular events occurring when BK channels operate in the body,” explains Riccardo Olcese.
The research team leader is an associate professor in the UCLA Department of Anesthesiology Division of Molecular Medicine. He is also a member of both the Cardiovascular Research Laboratory and Brain Research Institute at the David Geffen School of Medicine at the university.
“This is an exciting field of study and we hope that these findings will lead to a greater understanding of how this complex biological sensor operates,” study author Anoosh D. Javaherian adds. He is colleagues with Olcese, at the UCLA David Geffen School of Medicine.
 14 DAY TRIAL //
14 DAY TRIAL //