A group of investigators in the UK discovered a new process taking place during the early development stages of mammalian embryos. The finding could apply to humans as well, although a series of studies is needed to confirm or infirm this hypothesis.
This type of pioneering investigation is precisely what we need in order to shed light on some of the most complex processes going on around us. In order to achieve their results, experts at the University of Nottingham developed an artificial womb of sorts.
The device comes in the form of a soft polymer bowl that looks, feels and behaves just like the soft tissues inside the uterus. After fertilization, embryos affix themselves to the uterine walls in order to continue their development, and receive the nourishment they need.
The study team, coordinated by UN professor of tissue engineering Kevin Shakesheff, published details of the investigation in the February 14 online issue of the top journal Nature Communications, here.
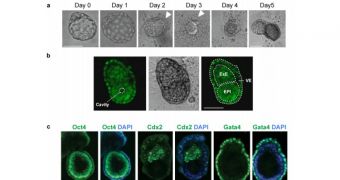
According to the research group, the study was mostly focused on figuring out what happens in a mouse embryo between the fourth and eighth day of development. For the first time ever, an embryo was grown outside the body of the mother for sufficient time to allow this to happen.
Until now, scientists could only observe fertilized embryos as they developed for four days, up to a developmental stage called a blastocyst. Beyond that time, the 64-cell embryo had to be implanted in a mother in order to survive.
“Using our unique materials and techniques we have been able to give our research colleagues a previously unseen view of the incredible behavior of cells at this vital stage of an embryo’s development,” Shakesheff says.
“We hope this work will unlock further secrets which could improve medical treatments that require tissues to regenerate and also open up more opportunities to improve” in-vitro fertilization treatments, the investigator goes on to say.
“In the future we hope to develop more technologies which will allow developmental biologists to understand how our tissue forms,” he concludes.
 14 DAY TRIAL //
14 DAY TRIAL //