Today, the problem of bioacumulation of heavy metals in the organisms is severe. Copper, cadmium, zinc, tin, mercury are found in the anthropic or human affected ecosystems in levels that are 10 times higher than in nature. Heavy metals abound around us in tiles (rich in cadmium and zinc), fertilizers (copper), pesticides (mercury), gas (lead) and so on.
Even stable systems, like high power lines, contaminate the surrounding terrains with copper ions. Sometimes, these contaminations lead to ecological catastrophes. But some organisms are able to resist and live in the conditions of high level accumulations of toxic heavy metals in their environment and even inside their bodies.
Snails, for example, can tolerate concentrations that kill other organisms. Around metallurgic plants, they were found to be well with 14 times the normal cadmium level, 6 times the zinc, and twice the lead. Other species can accumulate thousands of times the deadly amount for other species.
How do they cope with this?
First, we have to see how in general metal ions enter the cell. The ions pass through the fatty cell membrane in free form but especially combined with natural or artificial ligands present in the watery solution. Most ligands are not polarized and not electrically charged and form with the metal ions complex compounds named chelates.
Chelates have high penetration power, pass easily trough the fatty cell wall and introduce the uninvited "guests" inside the cell. For example the complex mercury-chlorine pierces through the cell barrier 20 times faster than the water and one million times easier than the sodium ion, a vital element for life.
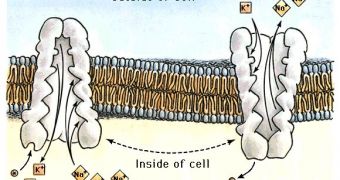
Another way employed by heavy ions to enter the cells is by parasitizing the cells' ion pumps. Bivalent metals, like cadmium and calcium, overlap in the calcium's natural circuitry, competing with it for entering the cell. Sodium is competed at pumps by cesium, a monovalent metal.
Small mineral inclusions, that appear on the cell wall in the presence of an excess of minerals, are "digested" with the walls, entering in the biological cycle this way.
Researchers looked at what happens with the toxic heavy metals looking at vital heavy metals, like iron and potassium. They have a special stocking system, that ensures the "good functioning" when normal supply with these compounds cannot be made. The chemical agent responsible for iron bioacumulation was found to be a protein called feritine. Its large molecule forms a perforated sphere assemble.
In the radial channels and inner space delimited by its subunits can be harbored up to 4,500 iron atoms. The feritine macromolecules are digested after few days by cell organelles named lysosomes, and the excess iron accumulating here likes oxides (hematite or siderite).
The "disintoxication" of the toxic heavy metals is made in a similar way. Here, the metalotioneine proteins act, with a small molecule and containing numerous sulphydric (-SH) groups, in which the hydrogen ion can be easily replaced by a metal ion. This way, the metallothioneins can easily bind toxic metal ions in complex combinations.
Their metabolisation leads to their sedimentation as insoluble salts like cadmium sulphide, which accumulate in the cell in concretions. In the invertebrates' organisms, these heavy ions are immobilized as insoluble phosphate and pyrophosphate salts.
In just a few hours, invertebrates exposed to high heavy metal levels presented no sign of them in their blood, and synthesized insoluble salts, deposed in successive layers, forming granules with the diameter of 1 to 10 microns. The granules contain both calcium and magnesium phosphates and heavy metal ions (manganese, lead, barium, tin, strontium, and so on). These mechanisms explain why clams and mussels are so efficient in cleaning contaminated waters without being killed.
Today, chelating agents are employed in the treatment of humans intoxications with radioactive lead or strontium. These agents, like in invertebrates, fix the dangerous heavy metals.
 14 DAY TRIAL //
14 DAY TRIAL //