Researchers at the University of California in Los Angles (UCLA) have recently determined how the HIV TAT cell-penetrating peptide is capable of breaching cellular defenses in order to deliver its cargo. The findings could be used to create highly-advanced, molecular drug delivery systems.
At this point, an important area of research is creating nanoscale systems for delivering drug particles to or inside cells. The chemicals may be loaded into spherical nanoparticles, for example, and released only when the target is reached.
But the ultimate goal in such studies – which is gaining the ability to actually penetrate the cell wall and enter the cellular medium – has thus far eluded researchers. What the UCLA team did in the new study was basically observe how the process occurs naturally.
Previous studies have shown that peptides can employ a wide variety of mechanisms in order to enter cells, including endocytosis, which is when the cell swells up and engulfs the whole molecule. Other peptides are capable of entering cells directly.
By studying cell-penetrating peptides (CPP), experts hope to be able to understand the basic principles of how they operate, and then replicate them in artificial, nanoparticle-based systems. The latter could be used in both in vitro and in vivo operations.
“The HIV TAT peptide is special. People discovered that one can attach almost anything to this peptide and it could drag it across the cell,” UCLA Henry Samueli School of Engineering and Applied Science professor of bioengineering and of chemistry and biochemistry Gerard Wong explains.
“So there are obvious beneficial drug-delivery and biotechnology applications,” adds the expert, who also holds an appointment with the California NanoSystems Institute at the university. Details of the work appear in this week's issue of the journal Proceedings of the National Academy of Science.
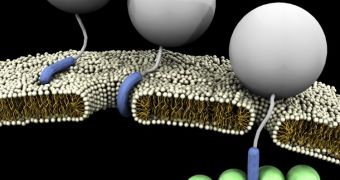
In the new study, the researchers observed how HIV TAT interacted with the membrane surrounding its target cells, as well as with the cell's actin cytoskeleton and certain receptors on the membrane that facilitated its task of getting through.
“Prior to this, people didn't really know how it all worked, but we found that the HIV TAT peptide is really kind of like a Swiss Army Knife molecule, in that it can interact very strongly with membranes, as well as with the cytoskeletons of cells,” Wong says.
“The second part wasn't well appreciated by the field,” concludes the expert, who is also the lead author of the PNAS paper. The US National Science Foundation (NSF) and the National Institutes of Health (NIH) funded this study.
 14 DAY TRIAL //
14 DAY TRIAL //