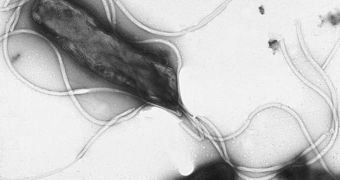
Scientists from the University of Illinois in Urbana-Champaign (UIUC) announce the discovery of a key mechanism that allows the bacteria Helicobacter pylori to kill tumor-suppressing proteins in target cells. H. pylori is the only microorganism known to science that can cause cancer, and so studying it may provide invaluable insight into the mechanisms the disease uses to spread and take over the body. The bacterium that the UIUC team focused on is particularly renowned for playing an active part in the emergence of stomach cancer.
H. pylori and stomach cancer have now been unequivocally linked. Writing in the latest issue of the esteemed medical journal Oncogene, the UIUC team details the previously-unknown mechanism the bacteria uses to create its characteristic inflammation of the stomach. This microorganism is among the select few that can endure in the harsh environment of the human stomach. About 66 percent of all people on the planet are infected with it, but only a few develop inflammations, ulcer, and then stomach cancer. Gastric cancer is the second leading cause of cancer deaths worldwide, statistics show.
The new investigation determined that the virulence protein called CagA, which CagA-positive H. pylori strains can develop, is one of the main weapons the pathogen uses in producing its effects. The molecule is inserted in the cells lining the stomach wall, where it begins to hijack various chemical pathways. Ultimately, the function of the cells is modified, and then the cell itself can become cancerous. The UIUC team also found that CagA can destroy the important tumor-suppressing protein called RUNX3, which plays a very important role in staving off the development of stomach cancer.
“Although emerging evidence suggests that RUNX3 is a tumor suppressor whose inactivation is involved in the initiation and progression of gastric cancer, the trigger for RUNX3 inactivation within gastric cells is largely unknown. The protein, RUNX3, is a transcription factor, so it activates different kinds of genes controlling cell growth and death. The first thing we wanted to see was whether H. pylori has any effect on the transcription activity of RUNX3,” explains team leader Lin-Feng Chen, who is a medical biochemistry professor at the University of Illinois.
“In fact, CagA alone is sufficient to down-regulate the RUNX3 transcription activity and reduce the expression of RUNX3, further supporting the importance of this bacterial protein in the genesis of gastric disease. This is the first time anybody has identified a unique domain within the amino-terminal region of the CagA protein, and it will help us to better understand how this oncogenic protein functions,” Chen concludes.
 14 DAY TRIAL //
14 DAY TRIAL //