Japanese scientists from the RIKEN Advanced Science Institute (ASI) are convinced that they've discovered a novel material technology, which they say could be used for the development of advanced fuel cells that will be more efficient than anything under development today.
The key to this achievement is the characterization of heterometallic molecular structures (HMS), which the Japanese team was the first-ever to do. Lightweight fuel cell technology would benefit extensively from such materials, if scientists manage to merge the two in a stable device.
One of the main advantages HMS would have over other materials is the fact that they are able to hold the same amount of hydrogen in a more compact package. This means that cars based on HMS fuel cells will either have longer range, or will be lighter than vehicles using other fuel cell types.
RIKEN chemists say that heterometallic molecular structures have this ability because they contain a previously-unknown mixture of rare-Earth elements and d-transition metals. However, this is a double-edged sword, since these chemicals tend to be very expensive, and also in short supplies.
Details of the new materials, and also about how they could be introduced in large-scale, practical applications, were published in the latest issue of the top scientific journal Nature Chemistry. The research team says that HMS have never-before-seen reactivity properties.
These traits make them exquisite choices for H2-based fuel cells, since these power packs need to be able to both store and release their energy supplies at great speeds, and on demand. The US Department of Energy (DOE), for example, has set strict guidelines on these values.
These limitations discourage compromises, and force scientists to create a first generation of hydrogen fuel cells capable of remaining in operation for years, until the next technological tier is available.
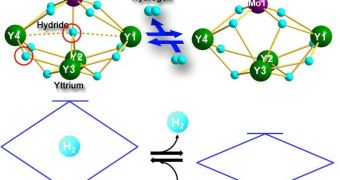
“While rare-earth / d-transition metal-type metallic hydride complexes have been studied in the past, the current research is the first to explore complexes with multiple rare earth atoms of the form Ln4MHn and with well-defined structures,” a RIKEN press release explains.
“Rare earth metal hydrides on their own […] do not undergo reversible hydrogen addition and release, the cornerstone of hydrogen storage. This becomes possible through the addition of a d-transition metal, in this case tungsten (W) or molybdenum (Mo),” the team adds.
HMS “complexes exhibit unique reactivity properties, pointing the way to new hydrogen storage techniques and promising environmentally-friendly solutions to today's pressing energy needs,” the document concludes.
 14 DAY TRIAL //
14 DAY TRIAL //