High-temperature superconductivity phonon theory has been recently declared officially dead, thus it is finally time to move on in the study of high-temperature superconductors. High-temperature superconductive materials experience zero electrical resistance at much higher temperatures than many other superconductors that experience similar behavior - usually somewhere around a few degrees above absolute zero.
Take cuprates for example - doped copper oxides start to superconduct at about 26 Kelvin (-248 degrees Celsius) but may remain in this state at temperatures as high as 148 Kelvin (-125 degrees Celsius), temperatures that can be easily achieved with liquid nitrogen. What triggers this behavior remains largely unexplained, but a new working theory developed by Cornell University researchers proposes that the phenomenon could be determined by a variation in the distances between atoms.
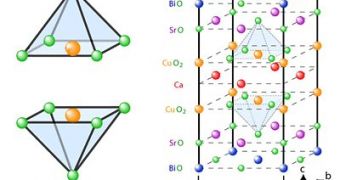
Cuprate superconductivity temperatures are in direct relation to the amount and mix of impurities used to dope the copper oxide material. According to Cornell researchers, copper and oxygen atoms form a pyramidal crystalline structure that can be altered through the addition of impurities so that the oxygen atom at the apex of the structure can be relocated to a lower or sideways position, thus changing the electron interactions between the atoms.
Using a scanning tunneling microscope, Cornell graduate student James Slezak along with professor of physics J.C. Seamus Davis revealed that cuprate crystalline structure variations could allow an electron interaction consistent with an electron pairing. While the oxygen atom at the apex is being pushed down, electron pairing is stronger - electron pairing is related to low-temperature superconductive materials, allowing the joined electrons to move freely through the crystalline structure, a process that is manifested on the large scale through zero electrical resistance.
"This proves that gluing the pairs together is a property of each crystal unit cell, not an overall property of the material", explained Davis.
The results of the experiment also show that electron pairing is most likely to occur in areas of the crystal next to the dopant atoms, located at random positions within the structure. "You don't need two different explanations", said Davis, since the squeezing and electron pairing phenomenon both occur at the same time.
 14 DAY TRIAL //
14 DAY TRIAL //