Materials can, for the most part, be classified in conductive and non-conductive, with the latter being possible to use as insulators depending on their, let's say, electrical permeability (or lack thereof). Carbyne, however, is both conductive and insulating.
It sounds oxymoronic, but it is, nonetheless true. Although, admittedly, the material cannot be both things at the same time. Instead, it switches between one and the other.
How? By stretching. Or being stretched by an outside force, like the curiosity-driven tools of a materials science researcher.
Carbyne is a sort of cousin to the well-known and famous graphene (which can be used for pretty much everything really). Made of carbon, it is comprised of single atomic chains, instead of two-dimensional atom layers.
It is also the strongest material in the world (yes, carbyne is better than even graphene in this). Nonetheless, it can be stretched.
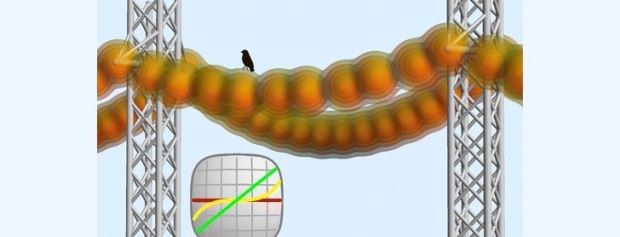
A team of scientists from Rice University found that stretching carbyne by just 3% caused it to change from conductor to insulator, due to quantum effects.
Chemically, carbyne is made of single and triple bonds alternated (1-3), but exists as both 1-3 and 2-2 bonds in “quantum reality.” Stretching it allows for the balance to swing to the 1-3 bond state, which alters the behavior of electrons.
In theory, this ability could allow carbyne to be used in nanoscale electronic devices and computers, where activation would happen through tugs and presses instead of electrical signals (transistors in general go from conducting to insulating all the time).
There is just one problem: carbyne is rare and very hard to make. Graphene is basically sheared pencil core material, but carbyne needs to be synthesized, and scientists haven't managed anything larger than 44-atom chains so far. Sure, the substance can sometimes be found in compressed graphite, but very rarely.
Maybe in the distant future someone will figure out a 3D printing technology capable of synthesizing the element in large quantities, but 3D printing isn't nearly close enough to that level. Sure, 3D printed graphene is already being tackled, but as we said, the material lends itself to production easily.
No doubt scientists will continue studying carbyne despite the comparative difficulties, but any practical applications will probably be postponed long enough for a more easily producible alternative to be discovered. It has happened before and it will happen again. If things go this route, said alternative will probably show up by 2020.

 14 DAY TRIAL //
14 DAY TRIAL //