Glioblastoma, the most common and deadly form of brain cancer, resists almost all treatments, and researchers from the Salk Institute for Biological Studies think the reason for this is the tumor cells' extreme flexibility.
Once they feel a life-threatening oxygen shortage, glioblastoma cells can turns into blood vessels, thus ensuring the continuity of nutrients supply, explained a research team led by Inder Verma, PhD.
This explains why cancer treatments that target angiogenesis* always fail in glioblastoma, and the findings could contribute to new drugs and different approaches.
Senior author Verma is a professor in the Laboratory of Genetics and holder of the Irwin and Joan Jacobs Chair in Exemplary Life Science, and he says that “this surprising effect of anti-angiogenic therapy with drugs such as Avastin tells us that we have to rethink glioblastoma combination therapy.
“Disrupting the formation of tumor blood vessels is not enough; we also have to prevent the conversion of tumor cells into blood vessels cells.”
Tumors need their own independent blood supply to grow, so many tumors overexpress growth factors – especially vascular endothelial growth factor (VEGF), to take new vasculature from existing blood vessels.
This is the reason for which scientists developed Avastin, a monoclonal antibody that intercepts VEGF, but the problem is that tumor cells often become even more aggressive after anti-angiogenic therapy, without anyone knowing why.
Verma explains that “in a recent phase II clinical trial, 60 percent of patients with glioblastoma responded to a combination of Avastin and Irinotecan, which directly interferes with the growth of cancer cells, but in most patients this effect was only transient.”
To find out what's happening, postdoctoral researcher and first author Yasushi Soda, PhD, used a mouse model of glioblastoma.
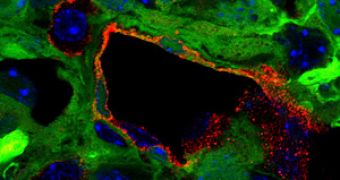
He said that “the tumors in these mice closely resemble glioblastomas, including the typically messy and highly permeable tumor vessels, which allowed us to study the tumor vasculature in great detail.”
These mice grow brain tumors a few months after being injected with viruses containing activated oncogenes and a marker gene, which causes all cells derived from tumor cells, to glow green under ultraviolet light.
After tracking the green glow under the microscope, the researchers understood what happened to tumor cells.
Once Soda looked at them, he discovered that nearly 30% of vascular endothelial cells (lining the interior surface of blood vessels) were green.
“This indicated to us that they most likely originated from tumor cells, “ the scientist said.
After several experiments, the scientists concluded that tumor-derived endothelial cells (TDECs) are not specific to mouse tumors, but they can also be found in glioblastoma human patients.
Soda added that “this was really strong evidence for us that glioblastoma cells routinely transdifferentiate into endothelial cells.”
The cells metamorphose because of low oxygen levels, or hypoxia, except that unlike normal vascular endothelial cells, TDECs don't need VEGF to form functional blood vessels.
Verma said that “once again, we are confronted with the versatility of tumor cells, which allows them to survive and thrive under adverse conditions.
“But as we learn more about tumors' molecular flexibility, we will be able to design novel, tailor-made combination therapies to combat deadly brain tumors.”
The findings are described by Verma's team in a feature article in this week's issue of the Proceedings of the National Academy of Sciences.
*Angiogenesis is the growth of a network of blood vessels that supplies nutrients and oxygen to cancerous tissues.
 14 DAY TRIAL //
14 DAY TRIAL //