Magnetocaloric materials are a new class of materials, designed for magnetic refrigeration, that use the magnetocaloric effect to attain extremely low temperatures (well below 1 kelvin), as well as the ranges used in common refrigerators, depending on the design of the system.
The magnetocaloric effect is a magneto-thermodynamic phenomenon in which a reversible change in temperature of a suitable material is caused by exposing the material to a changing magnetic field.
New information about these materials is now provided by researchers at the Advanced Photon Source at Argonne Laboratories, using the most powerful x-ray sources. These materials do not use greenhouse gases, thus having a great potential for environmentally friendly magnetic refrigeration systems.
How exactly do they achieve the cooling effect? They use magnetic fields to manipulate the degree of ordering (or entropy) of electronic or nuclear magnetic dipoles in order to reduce a material's temperature and allow the material to serve as a refrigerant.
One of the most notable examples of the magnetocaloric effect is in the chemical element gadolinium and some of its alloys. Gadolinium's temperature is observed to increase when it enters certain magnetic fields. When it leaves the magnetic field, the temperature returns to normal.
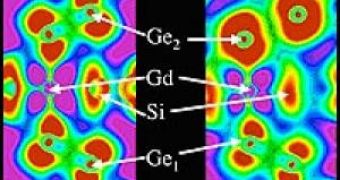
The most impressive display of this effect has been achieved using gadolinium-germanium-silicon alloys, that have an unusual coupling between the material's magnetism and chemical structure.
"This is surprising and important," said Argonne physicist Daniel Haskel, who led the research team. "Germanium was expected to be non-magnetic. Its magnetization is induced by the hybridization, or mixing, of otherwise non-magnetic germanium atomic orbitals with the magnetic gadolinium orbitals. This hybridization dramatically changes at the germanium-silicon bond-breaking transition, causing the destruction of magnetic ordering and leading to the giant magnetocaloric effect of these materials."
In fact, the results of the study showed that the magnetized germanium orbitals act as "magnetic bridges" in mediating the magnetic interactions across the distant gadolinium ions, thus making gadolinium the current material of choice for magnetic refrigeration near room temperature.
"As a result of this work we now have a better understanding of the role of nonmagnetic elements, such as germanium, in enhancing magnetic interactions between the rare-earth metals in these materials," said co-author and Ames Laboratory senior scientist Vitalij Pecharsky. "This discovery is counterintuitive, yet it opens up a range of exciting new opportunities towards the engineering of novel magnetic materials with predictable properties."
 14 DAY TRIAL //
14 DAY TRIAL //