A group of American investigators announces the creation of a new method for tracking GABA neurons in the human brain. Thus far, identifying these neural populations in a comprehensive manner has been nearly impossible in living creatures.
GABA neurons are important because they have the ability to release a potent neurotransmitter called gamma aminobutyric acid (GABA), which plays a critical role in determining the intensity of electrical signals being transmitted between excitatory neurons.
This makes GABA nerve cells a critical class of neurons. Neuroscientists have always felt the lack of an advanced set of data centered on these nerve cells, and this was one of the main reasons why the new study was conducted in the first place.
Experts from the Cold Spring Harbor Laboratory (CSHL) explain that excitatory neurons make up about 80 percent of all nerve cells in the cortex of mammals. Normal brain function would therefore be impossible with the constant influence of GABA, which regulates these interactions.
If neural excitation would not be inhibited by this neurotransmitter, then the brain would exist in a constant state of seizure, similar to what is seen during severe epilepsy episodes. Mapping GABA neurons' disposition in the brain is an effort that began more than a century ago.
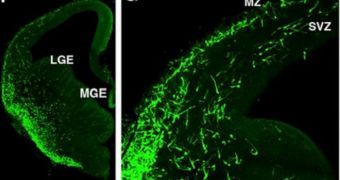
What the CSHL team did was create a method of tracking down these nerve cells. Called Cre driver lines, the technique relies on a research method called Cre-Lox recombination. It works by creating so-called genetic handles exclusively on cell types researchers tell it to.
Already, the investigators were able to map the connections that form between the roundworm C. elegans' 302 neurons. While this is a far cry from analyzing the billions of neurons in the mammalian brain, it does represent a significant step forward toward this goal.
The research team was led by CSHL professor Z. Josh Huang. The results of the new study, which he and his colleagues have been conducting for the past 5 years, are now published in the September 22 issue of the esteemed scientific journal Neuron.
Experts say that the work includes details about how to create genetically-engineered lines of mice, which express genetic triggers attached to GABA neurons, These triggers can be detected through medical imaging, enabling scientists to map the exact spread of these critical nerve cells.
“Optogenetics and retroviral labeling are wonderful techniques, but they are not, by themselves, cell-type specific. We’ve built a system that integrates all of these technologies, which can now be mobilized with exquisite specificity,” Huang concludes.
 14 DAY TRIAL //
14 DAY TRIAL //