A new study detailed in the March 5 issue of the prestigious New England Journal of Medicine showcases the first clinical trials centered around using a gene-editing technique to address infections with the human immunodeficiency virus (HIV). The tests revealed that the method can be safely applied to humans and showed that resilience to the viral agent can be increased significantly.
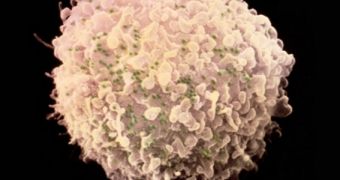
For this investigation, scientists with the University of Pennsylvania in Philadelphia, led by immunologists Carl June and Pablo Tebas, used a special class of enzymes called zinc-finger nucleases (ZFN) to target and destroy a particular gene found in the immune system cells of HIV patients.
The experiment included a total of 12 HIV patients, all of whom showed increased resilience to the viral agent at the end of the trial period. The team argues that these are the first major results to be obtained in the field of anti-HIV gene therapy, since the famous Berlin patient was cured of HIV.
John Rossi, a molecular biologist with the Beckman Research Institute in Duarte, California, says that Timothy Brown, an HIV patient at a hospital in Berlin, was treated for HIV via a bone-marrow transplant featuring stem cells that contained a mutated version of the CCR5 gene. The virus uses the proteins CCR5 codes for to infect T cells in the human immune system.
If a person has the mutated version of the CCR5 gene, then they are among the 7-10 percent of the world's population that is naturally resistant to HIV infections. Investigators around the world are now trying to extend this imperviousness to patients who have already been infected, Nature News reports.
What Tebas and June wanted to test for was a method of introducing CCR5 mutations in patients' cells without transplants, using gene-editing techniques. This is where ZFN molecules come in. The team first took blood samples from the 12 participants, and then cultured the blood cells in the lab.
After getting ahold of commercially-available ZFN molecules, they targeted the CCR5 genes. About a quarter of each patient's blood cells exhibited disrupted CCR5 expression. The team then injected the blood cells back into each test subject. At the end of the trials, all of them displayed elevated levels of immune T cells in their bloodstream, which means that the virus had a harder time killing them.
A subsequent study found that participants who were taken off their regular antiretroviral therapy continued to display elevated levels of T cells. At the same time, their HIV levels recovered slower than they normally would have. This observation led experts to conclude that the presence of HIV around the modified T cells was driving the latter to proliferate.
“They used HIV to help in its own demise. They throw the cells back at it and say, ‘Ha, now what'?” comments University of Southern California in Los Angeles gene therapy expert Paula Cannon, who was not a part of the research group.
 14 DAY TRIAL //
14 DAY TRIAL //