Scientists at the University of California in Los Angeles (UCLA) have recently developed a new type of nanostructures, which act in very much the same way fly paper does when it comes to catching, well, flies. The small structures are injected into the bloodstream, where they attract free-moving cancer cells, also known as circulating tumor cells, or CTCs. This allows researchers to pinpoint a large number of traits pertaining to the type of cancer that particular patient is suffering from. As a secondary benefit, the experts can also more easily follow the progress of the therapies they are using.
CTCs are the primary reason why people die of generalized cancer. This happens when the primary tumor becomes so large that it starts shedding cells, which make their way into the bloodstream, and then begin to set up colonies in other parts of the body as well. When they do so, they are vulnerable to interception, oncologists say. At this point, the only way of getting more data on the cancer plaguing a patient is to harvest metastatic solid biopsy samples. But this is very difficult to perform in the early stages of the disease, when identifying the correct site for the biopsy is tricky.
With the new method, scientists are essentially able to perform a liquid biopsy, which gives them more time to fight the condition, and also a better view of the disease as a whole. The UCLA effort is not the first to try this approach. Various methods of capturing the CTC have been devised, but the fly-paper one appears to yield the best results, by far. The team also adds that the amount of cancer cells harvested is also significantly increased in comparison with the yield of other techniques. Details of the accomplishment appear in the November issue of the respected scientific journal Angewandte Chemie.
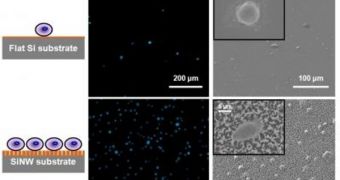
The scaffolding for the new structures is made up of a 1-by-2 centimeter silicon chip, which is embedded with tightly packed nanopillars. In laboratory settings, the nanoscale agents captured as much as 45 to 65 percent of all breast-cancer cells in their medium. “The nanopillar chip captured more than 10 times the amount of cells captured by the currently used flat structure,” UCLA David Geffen School of Medicine Crump Institute for Molecular Imaging postdoctoral researcher Dr. Shutao Wang explains.
The expert, who is the lead author of the new journal entry, also holds an appointment in the UCLA California NanoSystems Institute. “We hope that this platform can provide a convenient and cost-efficient alternative to CTC sorting by using mostly standard lab equipment,” Dr. Hsian-Rong Tseng, who is the senior author of the paper, adds. He is also an associate professor of molecular and medical pharmacology at both institutes.
 14 DAY TRIAL //
14 DAY TRIAL //