Scientists were given green light by the Australian government, in order to obtain embryonic stem cells out of cloned human embryos. The license and 7.200 human eggs were granted to Sydney IVF, an in vitro fertilization company.
Previously, the practice had been banned, but the ban was lifted in December 2006 by Australia's national parliament. Still, other uses than the therapeutic one or creating other kinds of embryos is restricted by law. Dr. John Findlay, chair of the NHMRC's (National Health and Medical Research Council) licensing committee told Reuters about monitoring Sydney IVF: "They have been given a license to do therapeutic cloning. They can go to the stage called blastocyst. They must stop at that point." That means the researchers are not licensed to reach the fetal stage. The blastocyst defines one of the earliest stages of the embryo, before it is implanted into the womb.
Since nobody has been able to extract embryonic stem cells from cloned human embryos before, this would be the world's first success. In 2004, Sydney IVF was the first company to obtain stem cells from IVF embryos, and since then, it has obtained and grown 10 other embryonic stem cell colonies using this procedure. Stem cells have been obtained in the past, though, using various other techniques.
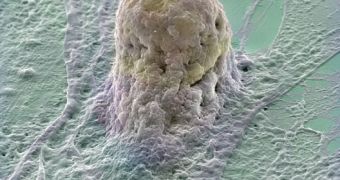
Somatic cell nuclear transfer refers to a technique where DNA from the nucleus of unfertilized eggs is removed and replaced by the nucleus of an adult cell (like skin cells). This procedure is used to develop cloned embryos from which embryonic stem cells are derived for therapeutic purposes, but it can also serve for reproductive cloning. From the several types of stem cells, the embryonic ones, obtained from several day-old embryos, are believed to be the best, as they can yield all the cell types of the body. In a first stage, the stem cells will be used for testing new medicines for illnesses like muscular dystrophy or Huntington's disease. Later on, body tissues matched to certain disease-suffering patients will be developed through the cloning process.
As expected, critics of the endeavor began to rise. One of them is David van Gend, director of Australians for Ethical Stem Cell Research. He spoke against the license, stating that the newly developed technology should compensate the need for therapeutic cloning. As he declared to a local radio, "We have regulations in Australia such that the abuses of cloning wouldn't happen here, we will not get live birth cloning. We won't get cloning right through to the fetal stage in order to use them for organ transplants, but if we teach the world how to clone you can be quite sure it will be used in less rigorous jurisdictions."
In reply, Sydney IVF stated that only the eggs that were not usable for IVF as they were immature or not properly fertilized, and especially, which donors had previously given their consent for, will be used for the research. The officials from the firm added that they would use three different cell types in order to develop the cloned embryos: embryonic stem ones, cumulus cells attached to the collected eggs, and skin cells.
 14 DAY TRIAL //
14 DAY TRIAL //