The world's first human model of progeria, a very rare progressive genetic disorder that causes children to age rapidly, has been developed by scientists from A*STAR’s Institute of Medical Biology (IMB) in Singapore and the University of Hong Kong’s Department of Medicine.
The model allowed the researchers to find out more about the mechanism of this disease, that affects one in eight million children.
The Hutchinson-Gilford Progeria Syndrome (HGPS), or simply progeria, begins in the child's first two years of life, and it is so rare, that since 1886, only around 130 cases of progeria have been recorded in scientific literature.
Kids with progeria look normal at birth, but by 12 months, they start having signs and symptoms, like skin changes and hair loss.
A child with progeria has an average life expectancy of 13 years, but there are some who die younger, and some that live 20 years or even longer.
Ultimately, most of them die as a result of heart problems or stroke, and the condition has no cure so far.
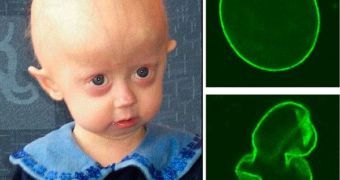
HGPS is caused by a mutation in a the gene encoding the protein lamin A, a very important component of the membrane surrounding the nucleus of a cell.
This mutation creates progerin, a shorter form of lamin A, which causes DNA damage and misshapen cell nuclei.
Prior to this research, only mouse models of the condition were available and as Professor Alan Colman said, “while mouse models of progeria have been informative, no one mouse model recapitulates all the symptoms seen in humans.
“Our human progeria model allows us to examine the pathology of the disease at a much closer resolution than previously possible.”
IMB’s Professors Alan Colman and Colin Stewart led the team, and used a new technique of deriving induced pluripotent stem (iPS) cells from cells of human progeria patients.
This way, they were able to trace and analyze the distinctive features of the disease, as it progresses in human cells.
The researchers identified two types of cells that were particularly affected by progeria – mesenchymal stem cells (MSCs) and vascular smooth muscle cells (VSMCs), meaning that a young patient has fewer MSCs and VSMCs than other children.
It looks like MSCs are very sensitive to a low oxygen environment and the lesser they are, the harder is for the tissues they created to renew themselves, thus accelerating the patient's symptoms of aging.
The same applies to VSMCs and it explains why their number in the patient’s heart vessels was low.
“This new study provides further evidence for the role of lamin processing in connective tissue function, as well as insights into the normal aging process,” said Professor Stewart.
“We hope to soon find new routes of intervention to treat this incurable disease.
“Such interventions may be of use in treating atherosclerosis in general, a condition afflicting many millions of individuals.”
The findings were published this month in the scientific journal, Cell Stem Cell.
 14 DAY TRIAL //
14 DAY TRIAL //