Officials with the US Food and Drug Administration (FDA) announced yesterday, March 14, that the first generic Lexapro (escitalopram) tablets had been approved for use in the United States, for the treatment of both anxiety and depression.
Both of these conditions can have debilitating consequences on patients' quality of life. In many instances, the disorders occur together, a fact that compounds their negative effects of people's health.
Anxiety and depression usually occur in episodes. Even if medication works in keeping one episode in check, a patient will most likely relapse several times during the course of their lifetimes, and medication may be required every single time.
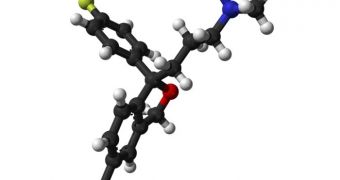
Escitalopram is a chemical developed by Danish international pharmaceutical company H. Lundbeck A/S, based on an earlier chemical called citalopram. The compound is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class.
Brand names under which it is or was available include Anxiset-E (on Indian markets) Lexapro, Cipralex, Seroplex, Lexamil, Lexam, and Entact. With the new decision, the FDA basically allows other manufacturers to produce antidepressant medication using a generic form of this substance.
Teva Pharmaceutical Industries/IVAX Pharmaceuticals has already gained FDA approval for the marketing of generic escitalopram tablets, in 5 milligram, 10 mg, and 20 mg strengths. But the Administration also found some side-effects associated with the drug.
These include insomnia, ejaculation disorder, nausea, increase in sweating, fatigue and drowsiness, and decreased libido. But depression and anxiety “can be disabling and prevent a person from doing every-day activities” anyway, Janet Woodcock, MD, explains.
“This medication is widely used by people who must manage their condition over time, so it is important to have affordable treatment options,” adds the official, who holds an appointment as the director of the FDA Center for Drug Evaluation and Research.
“Teva has been granted a 180-day period of generic drug exclusivity, which means that FDA cannot approve another generic version of escitalopram tablets before the end of that period,” a FDA press release explains.
“Generic drugs approved by FDA have the same high quality and strength as brand-name drugs. The generic manufacturing and packaging sites must pass the same quality standards as those of brand-name drugs,” the statement concludes.
 14 DAY TRIAL //
14 DAY TRIAL //