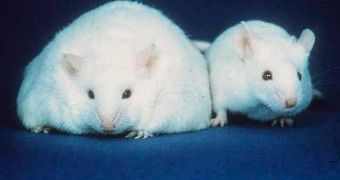
In a new study conducted on lab mice that were prone to becoming obese, researchers learned that transplanting immature nerve cells called neurons into the rodents' brains prevented the development of this dangerous condition.
The investigation could have significant applications in reducing obesity throughout the general population. In the developed world, and the United States specifically, obesity rates are through the roof. Statistically, 67 percent of all Americans are either overweight or obese.
Authorities are cataloging this as an epidemic, and say that it has been spreading rapidly for the last couple of decades. Preventing it would help save billions of dollars yearly, which the healthcare system currently spends of treating obesity and associated disorders.
The condition is among the most important risk factors for heart and cardiovascular diseases, as well as for diabetes. This means that the costs of treating obesity separately would be a lot smaller than those associated with handling other complications as well.
What is interesting about the new research is that it was not conducted specifically in order to test the efficiency of neural transplants on treating human obesity. Rather, this study was meant to show that immature neurons can be successfully integrated in existing, damaged neural pathways.
Through a successful integration, the pathway can be rebalanced and repaired, say experts at the Harvard University. The study – details of which appear in the November 24 online issue of the top journal Science – was led by neuroscientist Jeffrey Macklis, ScienceNow reports.
It could be that, in the future, treatments against obesity may be derived from this type of research. However, for the time being, experts are more focused on seeing how these cell therapies could be used to fight conditions such as amyotrophic lateral sclerosis, Parkinson's disease, and spinal injury.
“Reconstructing a circuit is going to require knitting together a number of specialized cells and not relying on the environment to do it. I'm still optimistic, but there's going to be a lot of intellectual heavy lifting,” scientist Evan Snyder comments.
He holds an appointment as a stem cell researcher at the Sanford-Burnham Medical Research Institute, in San Diego, California, but was not a part in the study. He is hopeful that the new data will indeed be useful, since “the work is obviously very carefully done.”
“These experiments say that if you get the neurons just right, it's possible for the adult brain to accept them, to wire them in, and have them fully functional,” Macklis adds. This is important when considering that new neurons might breach a gap in the spinal cord, restoring movement to the paralyzed.
 14 DAY TRIAL //
14 DAY TRIAL //