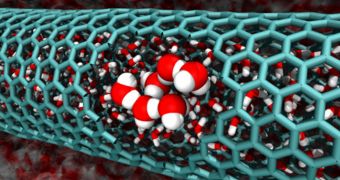
In a new study, experts determined that a measured of chaos called entropy explains the behavior of water at the nanoscale, where the liquid tends to flow spontaneously through carbon nanotubes.
In theory, this should not happen, because the vast network of hydrogen bonds that permeates water is extremely stable. Breaking this configuration requires energy, so the chemical was not expected to flow through such confined spaces by itself.
As a general rule, studies conducted at the nanoscale are revealing weird and unexpected behaviors in well-understood materials and chemicals, including water. However, the spontaneous flow was never fully explained until now.
Nanotubes are very tiny small tubes made out of graphite or graphene. They hold great promise for medicine, as well as for the emerging fields of nanofluidics (the smaller-scale cousin of microfluidics) and nanofiltration.
For the new investigation, California Institute of Technology (Caltech) researchers used a new approach for calculating the overall dynamics of water molecules in this scenario. They found that entropy – a physical measure for disorder and chaos in a system – is the key to explaining everything.
“It's a pretty surprising result. People normally focus on energy in this problem, not entropy,” William Goddard, the Caltech Charles and Mary Ferkel professor of chemistry, materials science, and applied physics, explains.
The expert holds an appointment as the director of the Materials and Process Simulation Center at the Institute. He explains that water should have no reason to “like” nanotubes, since entering such confined spaces would require hydrogen bonds to be broken.
“What we found is that it's actually a trade off. You lose some of that good energy stabilization from the bonding, but in the process you gain in entropy,” Goddard explains. The work is detailed in a recent issue of the esteemed journal Proceedings of the National Academy of Sciences (PNAS).
Goddard and postdoctoral scholar Tod Pascal studied carbon nanotubes with diameters between 0.8 and 2.7 nanometers, and found that water even enters the narrowest ones, with a diameter between 0.8 and 1 nanometers. The H2O molecules have to arrange themselves in a single file to accomplish this.
In order to verify the study results, investigators ran a simulation in which water was represented through all of its properties, other than the fact that it contained highly-ordered hydrogen bonds.
The computer model revealed that water was unable to enter the nanotubes. This further demonstrated that entropy is responsible for this amazing property.
 14 DAY TRIAL //
14 DAY TRIAL //