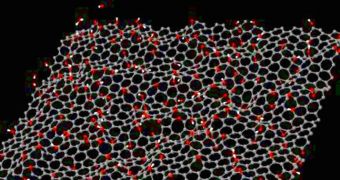
A team of investigators from the Brown University, led by expert Vivek Shenoy, a professor of engineering, announces the development of a new method for eliminating defects from graphene. In order for scientists and the electronics industry to take advantage of the amazing physical and chemical properties the single-atom-thick carbon compound has, it needs to be produced in a very pure manner. One process is called graphene-oxide reduction, but applying it results in imperfections. The technique developed at BU ensures that this production method results in more pure and defect-free graphene.
Graphene, and some of the chemicals that can be derived from it, “are materials people understand little about. The more we can understand their properties, the more (technological) possibilities that will be opened to us,” explains Shenoy. He is also the author of a new paper describing the findings, which appears in the latest issue of the respected scientific journal Nature Chemistry. The main thing that the purification methodology brings to the table is the use of hydrogen atoms for removing impurities, rather than heat, as was the case until now, ScienceDaily reports.
The defects appear due to the graphene production process. Most of the atoms on the sheets of graphene produced through this method are carbon. However, woven in the honeycomb-like structure are also atoms of hydrogen and oxygen. The latter form double bonds with carbon, and become unreactive. This means that they are generally very difficult to remove. The technique experts used thus far was applying heat to the graphene, which made some oxygen atoms come loose, bond with hydrogen, and form water. But not all oxygen could be removed through this method.
After using computer models to figure out the molecular structure of the graphene impurities, the research team, which also included scientists from the Rutgers University and the University of Texas-Dallas, determined that adding hydrogen atoms may in fact be the most efficient way to go. These atoms need to be placed in just the right amount at the correct location, but they are extremely well suited for removing “stubborn” oxygen. This investigation was funded by the US National Science Foundation (NSF) and the Semiconductor Research Corporation's Nanotechnology Research Initiative.
 14 DAY TRIAL //
14 DAY TRIAL //