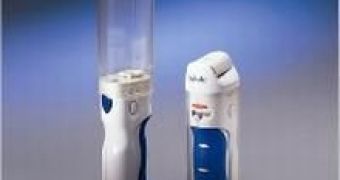
Britain's health officials rejected the inhaling of insulin, rather than administering it through injections. The drug Exubera, created by Pfizer, is designed as an alternative for daily insulin shots, but it costs ?1,100 per person a year, sufferers from diabetes still needing an injection at night.
Pfizer, the world's biggest pharmaceuticals group, declared on Tuesday that the decision of the National Institute for Health and Clinical Excellence (NICE) was "perverse and short-sighted".
January was the month in which Exubera won marketing approval in Europe and the United States for treating patients with type 1 and type 2 diabetes. NICE stated the drug did not offer sufficient benefits over conventional insulin injections to be worth the money.
The large price of the drug might be justified for people with severe fear of injections, but there was no way of identifying the people who would gain sufficient benefit. The insulin inhaler is the first non-injected option for insulin therapy since the discovery of the treatment for diabetes in the 1920s. Injections are used daily by 800,000 people in the U.K.
"Many people with diabetes will be deeply disappointed that they are being denied this alternative form of treatment. There is an urgent need for more research to support the improvements made by using inhaled insulin on quality of life," said a spokesman for Charity Diabetes U.K.
"Our review of the evidence indicated that inhaled insulin should not be recommended because it could not be proven to be more clinically or cost effective than existing treatments. The clinical experts we asked advised us that using injected insulin is not usually a concern for the majority of people with diabetes," concluded Andrea Sutcliffe, deputy chief executive at NICE.
 14 DAY TRIAL //
14 DAY TRIAL //